[English] 日本語
 Yorodumi
Yorodumi- PDB-6t40: Bovine enterovirus F3 in complex with a Cysteinylglycine dipeptide -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6t40 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bovine enterovirus F3 in complex with a Cysteinylglycine dipeptide | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords |  VIRUS / enterovirus F3 / enterovirus capsid assembly / VIRUS / enterovirus F3 / enterovirus capsid assembly /  glutathione / Cys-Gly dipeptide glutathione / Cys-Gly dipeptide | ||||||||||||
| Function / homology |  Function and homology information Function and homology information: /  picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / ribonucleoside triphosphate phosphatase activity / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / ribonucleoside triphosphate phosphatase activity / symbiont genome entry into host cell via pore formation in plasma membrane /  picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell ...: / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell ...: /  picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / ribonucleoside triphosphate phosphatase activity / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / ribonucleoside triphosphate phosphatase activity / symbiont genome entry into host cell via pore formation in plasma membrane /  picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell /  : / nucleoside-triphosphate phosphatase / protein complex oligomerization / monoatomic ion channel activity / : / nucleoside-triphosphate phosphatase / protein complex oligomerization / monoatomic ion channel activity /  RNA helicase activity / induction by virus of host autophagy / RNA helicase activity / induction by virus of host autophagy /  RNA-directed RNA polymerase / symbiont-mediated suppression of host gene expression / viral RNA genome replication / cysteine-type endopeptidase activity / RNA-directed RNA polymerase / symbiont-mediated suppression of host gene expression / viral RNA genome replication / cysteine-type endopeptidase activity /  RNA-dependent RNA polymerase activity / DNA-templated transcription / host cell nucleus / virion attachment to host cell / structural molecule activity / RNA-dependent RNA polymerase activity / DNA-templated transcription / host cell nucleus / virion attachment to host cell / structural molecule activity /  proteolysis / proteolysis /  RNA binding / RNA binding /  ATP binding / ATP binding /  metal ion binding metal ion bindingSimilarity search - Function | ||||||||||||
| Biological species |   Enterovirus F Enterovirus F | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.67 Å MOLECULAR REPLACEMENT / Resolution: 1.67 Å | ||||||||||||
 Authors Authors | Duyvesteyn, H.M.E. / Ren, J. / Walter, T.S. / Fry, E.E. / Stuart, D.I. | ||||||||||||
| Funding support |  United Kingdom, 3items United Kingdom, 3items
| ||||||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2020 Journal: Commun Biol / Year: 2020Title: Glutathione facilitates enterovirus assembly by binding at a druggable pocket. Authors: Duyvesteyn, H.M.E. / Ren, J. / Walter, T.S. / Fry, E.E. / Stuart, D.I. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6t40.cif.gz 6t40.cif.gz | 191.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6t40.ent.gz pdb6t40.ent.gz | 147.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6t40.json.gz 6t40.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/t4/6t40 https://data.pdbj.org/pub/pdb/validation_reports/t4/6t40 ftp://data.pdbj.org/pub/pdb/validation_reports/t4/6t40 ftp://data.pdbj.org/pub/pdb/validation_reports/t4/6t40 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6t48C  6t4cC  5osnS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | x 60
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-Protein , 4 types, 4 molecules ABCD
| #1: Protein | Mass: 30247.699 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Enterovirus F / References: UniProt: Q2LKZ0 Enterovirus F / References: UniProt: Q2LKZ0 |
|---|---|
| #2: Protein | Mass: 26757.965 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Enterovirus F / References: UniProt: Q2LKZ0 Enterovirus F / References: UniProt: Q2LKZ0 |
| #3: Protein | Mass: 27149.947 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Enterovirus F / References: UniProt: Q2LKZ0 Enterovirus F / References: UniProt: Q2LKZ0 |
| #4: Protein | Mass: 7705.456 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Enterovirus F / References: UniProt: Q2LKZ0 Enterovirus F / References: UniProt: Q2LKZ0 |
-Non-polymers , 8 types, 759 molecules 




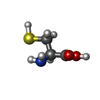









| #5: Chemical | ChemComp-GLY /  Glycine Glycine | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #6: Chemical | ChemComp-STE /  Stearic acid Stearic acid | ||||||||||
| #7: Chemical | ChemComp-K / #8: Chemical | ChemComp-SO4 /  Sulfate Sulfate#9: Chemical |  Glycerol Glycerol#10: Chemical | ChemComp-CYS / |  Cysteine Cysteine#11: Chemical |  Chloride Chloride#12: Water | ChemComp-HOH / |  Water Water |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / Details: 1.5 M Ammonium Sulfate, 0.1 M Tris at pH 8.5 / PH range: 8.5 |
|---|
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.976 Å / Beamline: I03 / Wavelength: 0.976 Å |
| Detector | Type: DECTRIS EIGER2 XE 16M / Detector: PIXEL / Date: May 12, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.976 Å / Relative weight: 1 : 0.976 Å / Relative weight: 1 |
| Reflection | Resolution: 1.67→19.92 Å / Num. obs: 2361113 / % possible obs: 99.4 % / Redundancy: 6.9 % / CC1/2: 0.994 / Rmerge(I) obs: 0.216 / Rpim(I) all: 0.089 / Rrim(I) all: 0.234 / Net I/σ(I): 7.2 |
| Reflection shell | Resolution: 1.67→1.7 Å / Redundancy: 5.2 % / Num. unique obs: 112481 / CC1/2: 0.4 / % possible all: 95.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5OSN Resolution: 1.67→19.92 Å / Rfactor Rfree error: 0.001 / Data cutoff high absF: 27373509.9 / Data cutoff low absF: 0 / Cross valid method: THROUGHOUT / σ(F): 0 / Details: BULK SOLVENT MODEL USED.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Bsol: 60.4101 Å2 / ksol: 0.4 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 22.4 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.67→19.92 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | NCS model details: CONSTR | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.67→1.77 Å / Rfactor Rfree error: 0.002 / Total num. of bins used: 6
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj













