[English] 日本語
 Yorodumi
Yorodumi- PDB-4q4y: Crystal structure of Coxsackievirus A24v soaked with Disialyllact... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4q4y | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Coxsackievirus A24v soaked with Disialyllacto-N-tetraose (DSLNT) | ||||||
 Components Components | (Coxsackievirus capsid protein ...) x 4 | ||||||
 Keywords Keywords |  VIRUS / Coxsackievirus A24v VIRUS / Coxsackievirus A24v | ||||||
| Function / homology |  Function and homology information Function and homology information : / : /  picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / ribonucleoside triphosphate phosphatase activity / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / ribonucleoside triphosphate phosphatase activity / symbiont genome entry into host cell via pore formation in plasma membrane /  picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell ... picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell ... : / : /  picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / ribonucleoside triphosphate phosphatase activity / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / ribonucleoside triphosphate phosphatase activity / symbiont genome entry into host cell via pore formation in plasma membrane /  picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell /  : / nucleoside-triphosphate phosphatase / protein complex oligomerization / monoatomic ion channel activity / : / nucleoside-triphosphate phosphatase / protein complex oligomerization / monoatomic ion channel activity /  RNA helicase activity / induction by virus of host autophagy / RNA helicase activity / induction by virus of host autophagy /  RNA-directed RNA polymerase / symbiont-mediated suppression of host gene expression / viral RNA genome replication / cysteine-type endopeptidase activity / RNA-directed RNA polymerase / symbiont-mediated suppression of host gene expression / viral RNA genome replication / cysteine-type endopeptidase activity /  RNA-dependent RNA polymerase activity / DNA-templated transcription / host cell nucleus / virion attachment to host cell / structural molecule activity / RNA-dependent RNA polymerase activity / DNA-templated transcription / host cell nucleus / virion attachment to host cell / structural molecule activity /  proteolysis / proteolysis /  RNA binding / RNA binding /  ATP binding / ATP binding /  metal ion binding metal ion bindingSimilarity search - Function | ||||||
| Biological species |   Coxsackievirus A24 Coxsackievirus A24 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.88 Å MOLECULAR REPLACEMENT / Resolution: 1.88 Å | ||||||
 Authors Authors | Zocher, G. / Stehle, T. | ||||||
 Citation Citation |  Journal: Plos Pathog. / Year: 2014 Journal: Plos Pathog. / Year: 2014Title: A sialic Acid binding site in a human picornavirus. Authors: Zocher, G. / Mistry, N. / Frank, M. / Hahnlein-Schick, I. / Ekstrom, J.O. / Arnberg, N. / Stehle, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4q4y.cif.gz 4q4y.cif.gz | 196.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4q4y.ent.gz pdb4q4y.ent.gz | 156.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4q4y.json.gz 4q4y.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/q4/4q4y https://data.pdbj.org/pub/pdb/validation_reports/q4/4q4y ftp://data.pdbj.org/pub/pdb/validation_reports/q4/4q4y ftp://data.pdbj.org/pub/pdb/validation_reports/q4/4q4y | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | x 60
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | x 5
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | x 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | x 15
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: (Schoenflies symbol : I (icosahedral : I (icosahedral )) )) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-Coxsackievirus capsid protein ... , 4 types, 4 molecules 1234
| #1: Protein | Mass: 34378.371 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Coxsackievirus A24 / References: UniProt: V9VEF3 Coxsackievirus A24 / References: UniProt: V9VEF3 |
|---|---|
| #2: Protein | Mass: 29817.412 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Coxsackievirus A24 / References: UniProt: V9VEF3 Coxsackievirus A24 / References: UniProt: V9VEF3 |
| #3: Protein | Mass: 26637.746 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Coxsackievirus A24 / References: UniProt: V9VEF3 Coxsackievirus A24 / References: UniProt: V9VEF3 |
| #4: Protein | Mass: 7319.045 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Coxsackievirus A24 / References: UniProt: V9VEF3 Coxsackievirus A24 / References: UniProt: V9VEF3 |
-Sugars , 1 types, 1 molecules 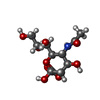
| #5: Sugar | ChemComp-SIA /  Sialic acid Sialic acid |
|---|
-Non-polymers , 6 types, 809 molecules 










| #6: Chemical |  1,6-Hexanediol 1,6-Hexanediol#7: Chemical | ChemComp-CA / #8: Chemical | ChemComp-CL /  Chloride Chloride#9: Chemical | ChemComp-MG / | #10: Chemical | ChemComp-MYR / |  Myristic acid Myristic acid#11: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 200 mM Magnesium chloride, 3.4 M 1,6-Hexanediol, 100 mM HEPES pH 7.5, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
|---|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.9763 Å / Beamline: I03 / Wavelength: 0.9763 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Dec 20, 2012 |
| Radiation | Monochromator: DCM / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9763 Å / Relative weight: 1 : 0.9763 Å / Relative weight: 1 |
| Reflection | Resolution: 1.88→49.78 Å / Num. all: 1626655 / Num. obs: 1552490 / % possible obs: 95.4 % / Observed criterion σ(F): -3 / Observed criterion σ(I): -3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 1.88→49.78 Å / Cor.coef. Fo:Fc: 0.958 / SU B: 2.084 / SU ML: 0.058 / ESU R: 0.021 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS MOLECULAR REPLACEMENT / Resolution: 1.88→49.78 Å / Cor.coef. Fo:Fc: 0.958 / SU B: 2.084 / SU ML: 0.058 / ESU R: 0.021 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 24.39 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.88→49.78 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller

























 PDBj
PDBj










