+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6p5s | ||||||
|---|---|---|---|---|---|---|---|
| Title | HIPK2 kinase domain bound to CX-4945 | ||||||
 Components Components | Homeodomain-interacting protein kinase 2 | ||||||
 Keywords Keywords | transferase/transferase inhibitor / Homeodomain Interacting Protein /  Kinase / p53 regulator / Kinase / p53 regulator /  Mitophagy / Mitophagy /  Fibrosis / Fibrosis /  NUCLEAR PROTEIN / transferase-transferase inhibitor complex NUCLEAR PROTEIN / transferase-transferase inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationlens induction in camera-type eye / iris morphogenesis / embryonic retina morphogenesis in camera-type eye / embryonic camera-type eye morphogenesis / retina layer formation / Physiological factors / PML body organization / voluntary musculoskeletal movement /  eye development / YAP1- and WWTR1 (TAZ)-stimulated gene expression ...lens induction in camera-type eye / iris morphogenesis / embryonic retina morphogenesis in camera-type eye / embryonic camera-type eye morphogenesis / retina layer formation / Physiological factors / PML body organization / voluntary musculoskeletal movement / eye development / YAP1- and WWTR1 (TAZ)-stimulated gene expression ...lens induction in camera-type eye / iris morphogenesis / embryonic retina morphogenesis in camera-type eye / embryonic camera-type eye morphogenesis / retina layer formation / Physiological factors / PML body organization / voluntary musculoskeletal movement /  eye development / YAP1- and WWTR1 (TAZ)-stimulated gene expression / SMAD protein signal transduction / adult walking behavior / eye development / YAP1- and WWTR1 (TAZ)-stimulated gene expression / SMAD protein signal transduction / adult walking behavior /  virion binding / anterior/posterior pattern specification / DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator / smoothened signaling pathway / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / positive regulation of transforming growth factor beta receptor signaling pathway / SMAD binding / Regulation of MECP2 expression and activity / negative regulation of BMP signaling pathway / intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / positive regulation of DNA binding / negative regulation of ubiquitin-dependent protein catabolic process / intrinsic apoptotic signaling pathway / virion binding / anterior/posterior pattern specification / DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator / smoothened signaling pathway / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / positive regulation of transforming growth factor beta receptor signaling pathway / SMAD binding / Regulation of MECP2 expression and activity / negative regulation of BMP signaling pathway / intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / positive regulation of DNA binding / negative regulation of ubiquitin-dependent protein catabolic process / intrinsic apoptotic signaling pathway /  erythrocyte differentiation / transforming growth factor beta receptor signaling pathway / SUMOylation of transcription cofactors / regulation of signal transduction by p53 class mediator / positive regulation of JNK cascade / peptidyl-threonine phosphorylation / neuron differentiation / PML body / positive regulation of DNA-binding transcription factor activity / cytoplasmic stress granule / RNA polymerase II transcription regulator complex / positive regulation of angiogenesis / transcription corepressor activity / positive regulation of protein binding / cellular response to hypoxia / peptidyl-serine phosphorylation / erythrocyte differentiation / transforming growth factor beta receptor signaling pathway / SUMOylation of transcription cofactors / regulation of signal transduction by p53 class mediator / positive regulation of JNK cascade / peptidyl-threonine phosphorylation / neuron differentiation / PML body / positive regulation of DNA-binding transcription factor activity / cytoplasmic stress granule / RNA polymerase II transcription regulator complex / positive regulation of angiogenesis / transcription corepressor activity / positive regulation of protein binding / cellular response to hypoxia / peptidyl-serine phosphorylation /  protein tyrosine kinase activity / neuron apoptotic process / Regulation of TP53 Activity through Phosphorylation / RNA polymerase II-specific DNA-binding transcription factor binding / negative regulation of neuron apoptotic process / cell population proliferation / protein tyrosine kinase activity / neuron apoptotic process / Regulation of TP53 Activity through Phosphorylation / RNA polymerase II-specific DNA-binding transcription factor binding / negative regulation of neuron apoptotic process / cell population proliferation /  transcription coactivator activity / transcription coactivator activity /  nuclear body / nuclear body /  non-specific serine/threonine protein kinase / non-specific serine/threonine protein kinase /  regulation of cell cycle / regulation of cell cycle /  protein kinase activity / positive regulation of protein phosphorylation / protein kinase activity / positive regulation of protein phosphorylation /  protein phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / positive regulation of cell population proliferation / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / protein phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / positive regulation of cell population proliferation / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II /  nucleoplasm / nucleoplasm /  ATP binding / ATP binding /  nucleus / nucleus /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.194 Å MOLECULAR REPLACEMENT / Resolution: 2.194 Å | ||||||
 Authors Authors | Agnew, C. / Liu, L. / Jura, N. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2019 Journal: J.Biol.Chem. / Year: 2019Title: The crystal structure of the protein kinase HIPK2 reveals a unique architecture of its CMGC-insert region. Authors: Agnew, C. / Liu, L. / Liu, S. / Xu, W. / You, L. / Yeung, W. / Kannan, N. / Jablons, D. / Jura, N. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6p5s.cif.gz 6p5s.cif.gz | 147.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6p5s.ent.gz pdb6p5s.ent.gz | 119.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6p5s.json.gz 6p5s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/p5/6p5s https://data.pdbj.org/pub/pdb/validation_reports/p5/6p5s ftp://data.pdbj.org/pub/pdb/validation_reports/p5/6p5s ftp://data.pdbj.org/pub/pdb/validation_reports/p5/6p5s | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 44977.051 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: HIPK2 / Production host: Homo sapiens (human) / Gene: HIPK2 / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: Q9H2X6,  non-specific serine/threonine protein kinase non-specific serine/threonine protein kinase |
|---|---|
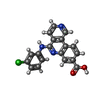
| #2: Chemical | ChemComp-3NG /  Silmitasertib Silmitasertib |
| #3: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.84 Å3/Da / Density % sol: 56.7 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / Details: 20% PEG 3350, 0.2 M KSCN |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 8.3.1 / Wavelength: 1.1111 Å / Beamline: 8.3.1 / Wavelength: 1.1111 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Oct 21, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.1111 Å / Relative weight: 1 : 1.1111 Å / Relative weight: 1 |
| Reflection | Resolution: 2.19→56.4 Å / Num. obs: 26108 / % possible obs: 99.98 % / Redundancy: 2 % / CC1/2: 0.998 / Rmerge(I) obs: 0.021 / Rpim(I) all: 0.021 / Rrim(I) all: 0.03 / Net I/σ(I): 17.63 |
| Reflection shell | Resolution: 2.19→2.27 Å / Redundancy: 2 % / Rmerge(I) obs: 0.24 / Mean I/σ(I) obs: 2.65 / Num. unique obs: 2564 / CC1/2: 0.857 / Rpim(I) all: 0.24 / Rrim(I) all: 0.35 / % possible all: 99.96 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 2.194→56.359 Å / SU ML: 0.23 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 25.28 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 2.194→56.359 Å / SU ML: 0.23 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 25.28 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.194→56.359 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -31.3253 Å / Origin y: 30.2717 Å / Origin z: -0.062 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller













 PDBj
PDBj