[English] 日本語
 Yorodumi
Yorodumi- PDB-6oqa: Crystal structure of CEP250 bound to FKBP12 in the presence of FK... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6oqa | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of CEP250 bound to FKBP12 in the presence of FK506-like novel natural product | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  CELL CYCLE / CELL CYCLE /  ISOMERASE / ISOMERASE /  FKBP12 / CEP250 / FKBP12 / CEP250 /  natural product / natural product /  ternary complex ternary complex | ||||||
| Function / homology |  Function and homology information Function and homology informationcentriole-centriole cohesion / detection of light stimulus involved in visual perception / protein localization to organelle / regulation of centriole-centriole cohesion / : /  macrolide binding / macrolide binding /  activin receptor binding / cytoplasmic side of membrane / positive regulation of protein localization to centrosome / activin receptor binding / cytoplasmic side of membrane / positive regulation of protein localization to centrosome /  transforming growth factor beta receptor binding ...centriole-centriole cohesion / detection of light stimulus involved in visual perception / protein localization to organelle / regulation of centriole-centriole cohesion / : / transforming growth factor beta receptor binding ...centriole-centriole cohesion / detection of light stimulus involved in visual perception / protein localization to organelle / regulation of centriole-centriole cohesion / : /  macrolide binding / macrolide binding /  activin receptor binding / cytoplasmic side of membrane / positive regulation of protein localization to centrosome / activin receptor binding / cytoplasmic side of membrane / positive regulation of protein localization to centrosome /  transforming growth factor beta receptor binding / TGFBR1 LBD Mutants in Cancer / signaling receptor inhibitor activity / type I transforming growth factor beta receptor binding / negative regulation of activin receptor signaling pathway / non-motile cilium assembly / heart trabecula formation / transforming growth factor beta receptor binding / TGFBR1 LBD Mutants in Cancer / signaling receptor inhibitor activity / type I transforming growth factor beta receptor binding / negative regulation of activin receptor signaling pathway / non-motile cilium assembly / heart trabecula formation /  terminal cisterna / terminal cisterna /  ryanodine receptor complex / ryanodine receptor complex /  I-SMAD binding / regulation of amyloid precursor protein catabolic process / I-SMAD binding / regulation of amyloid precursor protein catabolic process /  microtubule organizing center / protein maturation by protein folding / 'de novo' protein folding / ventricular cardiac muscle tissue morphogenesis / negative regulation of phosphoprotein phosphatase activity / microtubule organizing center / protein maturation by protein folding / 'de novo' protein folding / ventricular cardiac muscle tissue morphogenesis / negative regulation of phosphoprotein phosphatase activity /  FK506 binding / mTORC1-mediated signalling / TGF-beta receptor signaling activates SMADs / Calcineurin activates NFAT / FK506 binding / mTORC1-mediated signalling / TGF-beta receptor signaling activates SMADs / Calcineurin activates NFAT /  cilium assembly / photoreceptor outer segment / cilium assembly / photoreceptor outer segment /  regulation of immune response / protein peptidyl-prolyl isomerization / supramolecular fiber organization / heart morphogenesis / regulation of ryanodine-sensitive calcium-release channel activity / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / sarcoplasmic reticulum membrane / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / photoreceptor inner segment / regulation of immune response / protein peptidyl-prolyl isomerization / supramolecular fiber organization / heart morphogenesis / regulation of ryanodine-sensitive calcium-release channel activity / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / sarcoplasmic reticulum membrane / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / photoreceptor inner segment /  centriole / centriole /  T cell activation / AURKA Activation by TPX2 / ciliary basal body / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / T cell activation / AURKA Activation by TPX2 / ciliary basal body / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) /  sarcoplasmic reticulum / sarcoplasmic reticulum /  peptidylprolyl isomerase / peptidylprolyl isomerase /  peptidyl-prolyl cis-trans isomerase activity / calcium ion transmembrane transport / negative regulation of transforming growth factor beta receptor signaling pathway / peptidyl-prolyl cis-trans isomerase activity / calcium ion transmembrane transport / negative regulation of transforming growth factor beta receptor signaling pathway /  protein localization / Z disc / SARS-CoV-1 activates/modulates innate immune responses / protein localization / Z disc / SARS-CoV-1 activates/modulates innate immune responses /  regulation of protein localization / regulation of protein localization /  Regulation of PLK1 Activity at G2/M Transition / Regulation of PLK1 Activity at G2/M Transition /  protein folding / mitotic cell cycle / positive regulation of protein binding / protein refolding / positive regulation of canonical NF-kappaB signal transduction / Potential therapeutics for SARS / transmembrane transporter binding / amyloid fibril formation / protein domain specific binding / protein folding / mitotic cell cycle / positive regulation of protein binding / protein refolding / positive regulation of canonical NF-kappaB signal transduction / Potential therapeutics for SARS / transmembrane transporter binding / amyloid fibril formation / protein domain specific binding /  centrosome / centrosome /  protein kinase binding / perinuclear region of cytoplasm / protein-containing complex / extracellular exosome / protein kinase binding / perinuclear region of cytoplasm / protein-containing complex / extracellular exosome /  membrane / membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.2 Å molecular replacement / Resolution: 2.2 Å | ||||||
 Authors Authors | Lee, S.-J. / Shigdel, U.K. / Townson, S.A. / Verdine, G.L. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2020 Journal: Proc.Natl.Acad.Sci.USA / Year: 2020Title: Genomic discovery of an evolutionarily programmed modality for small-molecule targeting of an intractable protein surface. Authors: Shigdel, U.K. / Lee, S.J. / Sowa, M.E. / Bowman, B.R. / Robison, K. / Zhou, M. / Pua, K.H. / Stiles, D.T. / Blodgett, J.A.V. / Udwary, D.W. / Rajczewski, A.T. / Mann, A.S. / Mostafavi, S. / ...Authors: Shigdel, U.K. / Lee, S.J. / Sowa, M.E. / Bowman, B.R. / Robison, K. / Zhou, M. / Pua, K.H. / Stiles, D.T. / Blodgett, J.A.V. / Udwary, D.W. / Rajczewski, A.T. / Mann, A.S. / Mostafavi, S. / Hardy, T. / Arya, S. / Weng, Z. / Stewart, M. / Kenyon, K. / Morgenstern, J.P. / Pan, E. / Gray, D.C. / Pollock, R.M. / Fry, A.M. / Klausner, R.D. / Townson, S.A. / Verdine, G.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6oqa.cif.gz 6oqa.cif.gz | 187.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6oqa.ent.gz pdb6oqa.ent.gz | 147.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6oqa.json.gz 6oqa.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oq/6oqa https://data.pdbj.org/pub/pdb/validation_reports/oq/6oqa ftp://data.pdbj.org/pub/pdb/validation_reports/oq/6oqa ftp://data.pdbj.org/pub/pdb/validation_reports/oq/6oqa | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 8 molecules ABEFCDGH
| #1: Protein | Mass: 11967.705 Da / Num. of mol.: 4 / Source method: obtained synthetically / Source: (synth.)   Homo sapiens (human) / References: UniProt: P62942, Homo sapiens (human) / References: UniProt: P62942,  peptidylprolyl isomerase peptidylprolyl isomerase#2: Protein | Mass: 11429.831 Da / Num. of mol.: 4 / Fragment: UNP residues 2078-2175 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: CEP250, CEP2, CNAP1 / Production host: Homo sapiens (human) / Gene: CEP250, CEP2, CNAP1 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q9BV73 Escherichia coli (E. coli) / References: UniProt: Q9BV73 |
|---|
-Non-polymers , 9 types, 268 molecules 






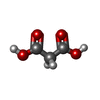









| #3: Chemical | ChemComp-60Z / ( #4: Chemical | ChemComp-EDO /  Ethylene glycol Ethylene glycol#5: Chemical | ChemComp-PEG /  Diethylene glycol Diethylene glycol#6: Chemical |  Polyethylene glycol Polyethylene glycol#7: Chemical | ChemComp-PGE /  Polyethylene glycol Polyethylene glycol#8: Chemical | ChemComp-PG4 /  Polyethylene glycol Polyethylene glycol#9: Chemical | ChemComp-MG / | #10: Chemical | ChemComp-MLA / |  Malonic acid Malonic acid#11: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.13 Å3/Da / Density % sol: 60.69 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop Details: 0.1 M HEPES, pH 7.0, 0.2 M sodium malonate, 21% PEG3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-G / Wavelength: 0.97856 Å / Beamline: 21-ID-G / Wavelength: 0.97856 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Dec 14, 2013 |
| Radiation | Monochromator: diamond(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.97856 Å / Relative weight: 1 : 0.97856 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→136.05 Å / Num. obs: 53422 / % possible obs: 99.5 % / Redundancy: 3.7 % / Net I/σ(I): 12.9 |
| Reflection shell | Resolution: 2.22→2.3 Å / Redundancy: 3.7 % / Mean I/σ(I) obs: 4.1 / Num. unique obs: 5345 / % possible all: 99.9 |
-Phasing
Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 2.2→136.05 Å / Cor.coef. Fo:Fc: 0.951 / Cor.coef. Fo:Fc free: 0.928 / SU B: 5.519 / SU ML: 0.142 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.249 / ESU R Free: 0.21 MOLECULAR REPLACEMENT / Resolution: 2.2→136.05 Å / Cor.coef. Fo:Fc: 0.951 / Cor.coef. Fo:Fc free: 0.928 / SU B: 5.519 / SU ML: 0.142 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.249 / ESU R Free: 0.21 Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 157.52 Å2 / Biso mean: 48.317 Å2 / Biso min: 22.03 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.2→136.05 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.203→2.26 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller







 PDBj
PDBj
















