[English] 日本語
 Yorodumi
Yorodumi- PDB-6enm: Crystal structure of MMP12 in complex with hydroxamate inhibitor ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6enm | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of MMP12 in complex with hydroxamate inhibitor LP168. | ||||||
 Components Components | Macrophage metalloelastase | ||||||
 Keywords Keywords |  HYDROLASE / metzincin / carboxylate inhibitor alternative zinc-binding groups HYDROLASE / metzincin / carboxylate inhibitor alternative zinc-binding groups | ||||||
| Function / homology |  Function and homology information Function and homology information macrophage elastase / negative regulation of endothelial cell-matrix adhesion via fibronectin / bronchiole development / positive regulation of epithelial cell proliferation involved in wound healing / elastin catabolic process / regulation of defense response to virus by host / positive regulation of type I interferon-mediated signaling pathway / macrophage elastase / negative regulation of endothelial cell-matrix adhesion via fibronectin / bronchiole development / positive regulation of epithelial cell proliferation involved in wound healing / elastin catabolic process / regulation of defense response to virus by host / positive regulation of type I interferon-mediated signaling pathway /  wound healing, spreading of epidermal cells / negative regulation of type I interferon-mediated signaling pathway / lung alveolus development ... wound healing, spreading of epidermal cells / negative regulation of type I interferon-mediated signaling pathway / lung alveolus development ... macrophage elastase / negative regulation of endothelial cell-matrix adhesion via fibronectin / bronchiole development / positive regulation of epithelial cell proliferation involved in wound healing / elastin catabolic process / regulation of defense response to virus by host / positive regulation of type I interferon-mediated signaling pathway / macrophage elastase / negative regulation of endothelial cell-matrix adhesion via fibronectin / bronchiole development / positive regulation of epithelial cell proliferation involved in wound healing / elastin catabolic process / regulation of defense response to virus by host / positive regulation of type I interferon-mediated signaling pathway /  wound healing, spreading of epidermal cells / negative regulation of type I interferon-mediated signaling pathway / lung alveolus development / positive regulation of interferon-alpha production / response to amyloid-beta / Collagen degradation / collagen catabolic process / extracellular matrix disassembly / core promoter sequence-specific DNA binding / wound healing, spreading of epidermal cells / negative regulation of type I interferon-mediated signaling pathway / lung alveolus development / positive regulation of interferon-alpha production / response to amyloid-beta / Collagen degradation / collagen catabolic process / extracellular matrix disassembly / core promoter sequence-specific DNA binding /  collagen binding / Degradation of the extracellular matrix / extracellular matrix organization / collagen binding / Degradation of the extracellular matrix / extracellular matrix organization /  extracellular matrix / extracellular matrix /  metalloendopeptidase activity / cellular response to virus / protein import into nucleus / metalloendopeptidase activity / cellular response to virus / protein import into nucleus /  endopeptidase activity / sequence-specific DNA binding / serine-type endopeptidase activity / endopeptidase activity / sequence-specific DNA binding / serine-type endopeptidase activity /  calcium ion binding / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / calcium ion binding / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II /  proteolysis / proteolysis /  extracellular space / zinc ion binding / extracellular region / extracellular space / zinc ion binding / extracellular region /  nucleus / nucleus /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.59 Å MOLECULAR REPLACEMENT / Resolution: 1.59 Å | ||||||
 Authors Authors | Vera, L. / Nuti, E. / Rossello, A. / Stura, E.A. | ||||||
 Citation Citation |  Journal: J. Med. Chem. / Year: 2018 Journal: J. Med. Chem. / Year: 2018Title: Development of Thioaryl-Based Matrix Metalloproteinase-12 Inhibitors with Alternative Zinc-Binding Groups: Synthesis, Potentiometric, NMR, and Crystallographic Studies. Authors: Nuti, E. / Cuffaro, D. / Bernardini, E. / Camodeca, C. / Panelli, L. / Chaves, S. / Ciccone, L. / Tepshi, L. / Vera, L. / Orlandini, E. / Nencetti, S. / Stura, E.A. / Santos, M.A. / Dive, V. / Rossello, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6enm.cif.gz 6enm.cif.gz | 89.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6enm.ent.gz pdb6enm.ent.gz | 67.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6enm.json.gz 6enm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/en/6enm https://data.pdbj.org/pub/pdb/validation_reports/en/6enm ftp://data.pdbj.org/pub/pdb/validation_reports/en/6enm ftp://data.pdbj.org/pub/pdb/validation_reports/en/6enm | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6eknC  6elaC  6eoxC  6esmC  4h76S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 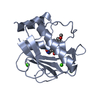
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 17615.670 Da / Num. of mol.: 2 / Mutation: F171D Source method: isolated from a genetically manipulated source Details: matrix metalloprotein 12 / Source: (gene. exp.)   Homo sapiens (human) / Cell: macrophage / Gene: MMP12, HME / Production host: Homo sapiens (human) / Cell: macrophage / Gene: MMP12, HME / Production host:   Escherichia coli (E. coli) / References: UniProt: P39900, Escherichia coli (E. coli) / References: UniProt: P39900,  macrophage elastase macrophage elastase#2: Chemical |   Mass: 397.444 Da / Num. of mol.: 2 / Mutation: F171D Mass: 397.444 Da / Num. of mol.: 2 / Mutation: F171DSource method: isolated from a genetically manipulated source Formula: C21H19NO5S / Details: matrix metalloprotein 12 / Source: (gene. exp.)   Homo sapiens (human) / Cell: macrophage / Production host: Homo sapiens (human) / Cell: macrophage / Production host:   Escherichia coli (E. coli) / References: Escherichia coli (E. coli) / References:  macrophage elastase macrophage elastase#3: Chemical | ChemComp-ZN / #4: Chemical | ChemComp-CA / #5: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2 Å3/Da / Density % sol: 38.53 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: Drops: 1 micro-L hMMP12 at 927 micro-M + 10 mM acetohydroxamic acid, LP168 at 1 milli-M and 1 micro-L precipitant. Precipitant: 17% PEG 20K, 0.2 M imidazole malate, pH 8.5, 0.250 M NaCl ...Details: Drops: 1 micro-L hMMP12 at 927 micro-M + 10 mM acetohydroxamic acid, LP168 at 1 milli-M and 1 micro-L precipitant. Precipitant: 17% PEG 20K, 0.2 M imidazole malate, pH 8.5, 0.250 M NaCl cryoprotectant: 27% PEG8K, 15% MPEG550, 10% glycerol, 90mM Tris-HCl, pH 8.0 PH range: 8.0-8.5 / Temp details: cooled incubator |
-Data collection
| Diffraction | Mean temperature: 100 K / Ambient temp details: cryostream |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.9184 Å / Beamline: PROXIMA 1 / Wavelength: 0.9184 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Dec 20, 2010 / Details: mirrors |
| Radiation | Monochromator: Si 111 CHANNEL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9184 Å / Relative weight: 1 : 0.9184 Å / Relative weight: 1 |
| Reflection | Resolution: 1.59→47.91 Å / Num. obs: 38794 / % possible obs: 99.7 % / Observed criterion σ(I): -3 / Redundancy: 7.93 % / Rsym value: 0.123 / Net I/σ(I): 12.1 |
| Reflection shell | Resolution: 1.59→1.68 Å / Redundancy: 8.11 % / Mean I/σ(I) obs: 2.9 / Rsym value: 0.85 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4H76 Resolution: 1.59→47.91 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.96 / SU B: 1.884 / SU ML: 0.065 / Cross valid method: THROUGHOUT / ESU R: 0.098 / ESU R Free: 0.093 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 18.65 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.59→47.91 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj








