+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6aq7 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of POM6 FAB fragment complexed with mouse PrPc | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  IMMUNE SYSTEM / IMMUNE SYSTEM /  Prion / Prion /  antibody / antibody /  chaperone / antigen-antibody chaperone / antigen-antibody | ||||||
| Function / homology |  Function and homology information Function and homology informationInsertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of amyloid precursor protein catabolic process /  lamin binding / regulation of glutamate receptor signaling pathway / regulation of calcium ion import across plasma membrane / aspartic-type endopeptidase inhibitor activity / lamin binding / regulation of glutamate receptor signaling pathway / regulation of calcium ion import across plasma membrane / aspartic-type endopeptidase inhibitor activity /  glycosaminoglycan binding / ATP-dependent protein binding / regulation of potassium ion transmembrane transport / negative regulation of interleukin-17 production ...Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of amyloid precursor protein catabolic process / glycosaminoglycan binding / ATP-dependent protein binding / regulation of potassium ion transmembrane transport / negative regulation of interleukin-17 production ...Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of amyloid precursor protein catabolic process /  lamin binding / regulation of glutamate receptor signaling pathway / regulation of calcium ion import across plasma membrane / aspartic-type endopeptidase inhibitor activity / lamin binding / regulation of glutamate receptor signaling pathway / regulation of calcium ion import across plasma membrane / aspartic-type endopeptidase inhibitor activity /  glycosaminoglycan binding / ATP-dependent protein binding / regulation of potassium ion transmembrane transport / negative regulation of interleukin-17 production / negative regulation of dendritic spine maintenance / type 5 metabotropic glutamate receptor binding / cupric ion binding / nucleobase-containing compound metabolic process / response to copper ion / negative regulation of calcineurin-NFAT signaling cascade / negative regulation of interleukin-2 production / negative regulation of T cell receptor signaling pathway / activation of protein kinase activity / cuprous ion binding / negative regulation of amyloid-beta formation / negative regulation of activated T cell proliferation / response to amyloid-beta / : / negative regulation of type II interferon production / intracellular copper ion homeostasis / negative regulation of long-term synaptic potentiation / positive regulation of protein targeting to membrane / side of membrane / response to cadmium ion / regulation of peptidyl-tyrosine phosphorylation / glycosaminoglycan binding / ATP-dependent protein binding / regulation of potassium ion transmembrane transport / negative regulation of interleukin-17 production / negative regulation of dendritic spine maintenance / type 5 metabotropic glutamate receptor binding / cupric ion binding / nucleobase-containing compound metabolic process / response to copper ion / negative regulation of calcineurin-NFAT signaling cascade / negative regulation of interleukin-2 production / negative regulation of T cell receptor signaling pathway / activation of protein kinase activity / cuprous ion binding / negative regulation of amyloid-beta formation / negative regulation of activated T cell proliferation / response to amyloid-beta / : / negative regulation of type II interferon production / intracellular copper ion homeostasis / negative regulation of long-term synaptic potentiation / positive regulation of protein targeting to membrane / side of membrane / response to cadmium ion / regulation of peptidyl-tyrosine phosphorylation /  inclusion body / cellular response to copper ion / neuron projection maintenance / protein sequestering activity / inclusion body / cellular response to copper ion / neuron projection maintenance / protein sequestering activity /  tubulin binding / negative regulation of protein phosphorylation / molecular condensate scaffold activity / molecular function activator activity / positive regulation of protein localization to plasma membrane / protein destabilization / protein homooligomerization / negative regulation of DNA-binding transcription factor activity / tubulin binding / negative regulation of protein phosphorylation / molecular condensate scaffold activity / molecular function activator activity / positive regulation of protein localization to plasma membrane / protein destabilization / protein homooligomerization / negative regulation of DNA-binding transcription factor activity /  terminal bouton / cellular response to amyloid-beta / terminal bouton / cellular response to amyloid-beta /  regulation of protein localization / positive regulation of peptidyl-tyrosine phosphorylation / positive regulation of neuron apoptotic process / cellular response to xenobiotic stimulus / regulation of protein localization / positive regulation of peptidyl-tyrosine phosphorylation / positive regulation of neuron apoptotic process / cellular response to xenobiotic stimulus /  signaling receptor activity / signaling receptor activity /  amyloid-beta binding / protein-folding chaperone binding / amyloid-beta binding / protein-folding chaperone binding /  microtubule binding / microtubule binding /  nuclear membrane / nuclear membrane /  protease binding / response to oxidative stress / mitochondrial outer membrane / transmembrane transporter binding / protease binding / response to oxidative stress / mitochondrial outer membrane / transmembrane transporter binding /  postsynaptic density / molecular adaptor activity / learning or memory / postsynaptic density / molecular adaptor activity / learning or memory /  membrane raft / copper ion binding / intracellular membrane-bounded organelle / membrane raft / copper ion binding / intracellular membrane-bounded organelle /  dendrite / protein-containing complex binding / negative regulation of apoptotic process / dendrite / protein-containing complex binding / negative regulation of apoptotic process /  Golgi apparatus / Golgi apparatus /  cell surface / cell surface /  endoplasmic reticulum / endoplasmic reticulum /  membrane / identical protein binding / membrane / identical protein binding /  metal ion binding / metal ion binding /  plasma membrane / plasma membrane /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.83 Å MOLECULAR REPLACEMENT / Resolution: 1.83 Å | ||||||
 Authors Authors | Baral, P.K. / Swayampakula, M. / James, M.N.G. | ||||||
| Funding support |  Canada, 1items Canada, 1items
| ||||||
 Citation Citation |  Journal: FEBS J. / Year: 2018 Journal: FEBS J. / Year: 2018Title: Structural characterization of POM6 Fab and mouse prion protein complex identifies key regions for prions conformational conversion. Authors: Baral, P.K. / Swayampakula, M. / Aguzzi, A. / James, M.N.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6aq7.cif.gz 6aq7.cif.gz | 132.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6aq7.ent.gz pdb6aq7.ent.gz | 99.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6aq7.json.gz 6aq7.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/aq/6aq7 https://data.pdbj.org/pub/pdb/validation_reports/aq/6aq7 ftp://data.pdbj.org/pub/pdb/validation_reports/aq/6aq7 ftp://data.pdbj.org/pub/pdb/validation_reports/aq/6aq7 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4j8rS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 14481.103 Da / Num. of mol.: 1 / Fragment: UNP residues 127-225 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Gene: Prnp, Prn-p, Prp / Production host: Mus musculus (house mouse) / Gene: Prnp, Prn-p, Prp / Production host:   Escherichia coli (E. coli) / References: UniProt: P04925 Escherichia coli (E. coli) / References: UniProt: P04925 |
|---|
-Antibody , 2 types, 2 molecules HL
| #2: Antibody | Mass: 23203.207 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Cell line (production host): hybridoma / Production host: Mus musculus (house mouse) / Cell line (production host): hybridoma / Production host:   Mus musculus (house mouse) Mus musculus (house mouse) |
|---|---|
| #3: Antibody | Mass: 23788.387 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Cell line (production host): hybridoma / Production host: Mus musculus (house mouse) / Cell line (production host): hybridoma / Production host:   Mus musculus (house mouse) Mus musculus (house mouse) |
-Non-polymers , 3 types, 463 molecules 
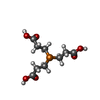



| #4: Chemical |  Glycerol Glycerol#5: Chemical | ChemComp-TCE / |  TCEP TCEP#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 50.88 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 0.2M Sodium malonate pH 7.0, 12% PEG8000 along with 5mM TCEP, 5mM Praseodymium acetate and ethanol |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CLSI CLSI  / Beamline: 08B1-1 / Wavelength: 0.97949 Å / Beamline: 08B1-1 / Wavelength: 0.97949 Å |
| Detector | Type: RAYONIX MX-300 / Detector: CCD / Date: Mar 21, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.97949 Å / Relative weight: 1 : 0.97949 Å / Relative weight: 1 |
| Reflection | Resolution: 1.83→48 Å / Num. obs: 54971 / % possible obs: 99.8 % / Redundancy: 9.3 % / Net I/σ(I): 9.29 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4J8R Resolution: 1.83→48 Å / SU ML: 0.27 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 23.97
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.83→48 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj






