[English] 日本語
 Yorodumi
Yorodumi- PDB-5x4l: Crystal structure of the UBX domain of human UBXD7 in complex wit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5x4l | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the UBX domain of human UBXD7 in complex with p97 N domain | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE/PROTEIN BINDING / UBXD7 / p97 / HYDROLASE-PROTEIN BINDING complex | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of Lys63-specific deubiquitinase activity / flavin adenine dinucleotide catabolic process / positive regulation of oxidative phosphorylation / VCP-NSFL1C complex / protein-DNA covalent cross-linking repair / endoplasmic reticulum stress-induced pre-emptive quality control / endosome to lysosome transport via multivesicular body sorting pathway / cellular response to arsenite ion / Derlin-1 retrotranslocation complex / BAT3 complex binding ...positive regulation of Lys63-specific deubiquitinase activity / flavin adenine dinucleotide catabolic process / positive regulation of oxidative phosphorylation / VCP-NSFL1C complex / protein-DNA covalent cross-linking repair / endoplasmic reticulum stress-induced pre-emptive quality control / endosome to lysosome transport via multivesicular body sorting pathway / cellular response to arsenite ion / Derlin-1 retrotranslocation complex / BAT3 complex binding / positive regulation of protein K63-linked deubiquitination / deubiquitinase activator activity / mitotic spindle disassembly / VCP-NPL4-UFD1 AAA ATPase complex /  aggresome assembly / NADH metabolic process / regulation of protein localization to chromatin / aggresome assembly / NADH metabolic process / regulation of protein localization to chromatin /  vesicle-fusing ATPase / cellular response to misfolded protein / vesicle-fusing ATPase / cellular response to misfolded protein /  : / stress granule disassembly / K48-linked polyubiquitin modification-dependent protein binding / positive regulation of mitochondrial membrane potential / negative regulation of protein localization to chromatin / ERAD pathway / ubiquitin-modified protein reader activity / retrograde protein transport, ER to cytosol / : / stress granule disassembly / K48-linked polyubiquitin modification-dependent protein binding / positive regulation of mitochondrial membrane potential / negative regulation of protein localization to chromatin / ERAD pathway / ubiquitin-modified protein reader activity / retrograde protein transport, ER to cytosol /  regulation of aerobic respiration / regulation of aerobic respiration /  ATPase complex / regulation of synapse organization / ubiquitin-specific protease binding / positive regulation of ATP biosynthetic process / ubiquitin-like protein ligase binding / autophagosome maturation / RHOH GTPase cycle / polyubiquitin modification-dependent protein binding / HSF1 activation / ATPase complex / regulation of synapse organization / ubiquitin-specific protease binding / positive regulation of ATP biosynthetic process / ubiquitin-like protein ligase binding / autophagosome maturation / RHOH GTPase cycle / polyubiquitin modification-dependent protein binding / HSF1 activation /  translesion synthesis / endoplasmic reticulum to Golgi vesicle-mediated transport / MHC class I protein binding / translesion synthesis / endoplasmic reticulum to Golgi vesicle-mediated transport / MHC class I protein binding /  Protein methylation / interstrand cross-link repair / negative regulation of smoothened signaling pathway / : / Attachment and Entry / ATP metabolic process / endoplasmic reticulum unfolded protein response / Protein methylation / interstrand cross-link repair / negative regulation of smoothened signaling pathway / : / Attachment and Entry / ATP metabolic process / endoplasmic reticulum unfolded protein response /  lipid droplet / lipid droplet /  proteasome complex / viral genome replication / Josephin domain DUBs / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / proteasome complex / viral genome replication / Josephin domain DUBs / N-glycan trimming in the ER and Calnexin/Calreticulin cycle /  ubiquitin binding / proteasomal protein catabolic process / ubiquitin binding / proteasomal protein catabolic process /  ADP binding / Hh mutants are degraded by ERAD / positive regulation of protein-containing complex assembly / Defective CFTR causes cystic fibrosis / ADP binding / Hh mutants are degraded by ERAD / positive regulation of protein-containing complex assembly / Defective CFTR causes cystic fibrosis /  macroautophagy / Hedgehog ligand biogenesis / Translesion Synthesis by POLH / ABC-family proteins mediated transport / establishment of protein localization / macroautophagy / Hedgehog ligand biogenesis / Translesion Synthesis by POLH / ABC-family proteins mediated transport / establishment of protein localization /  autophagy / Aggrephagy / cytoplasmic stress granule / positive regulation of non-canonical NF-kappaB signal transduction / positive regulation of protein catabolic process / positive regulation of canonical Wnt signaling pathway / activation of cysteine-type endopeptidase activity involved in apoptotic process / azurophil granule lumen / KEAP1-NFE2L2 pathway / double-strand break repair / Ovarian tumor domain proteases / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / E3 ubiquitin ligases ubiquitinate target proteins / site of double-strand break / cellular response to heat / autophagy / Aggrephagy / cytoplasmic stress granule / positive regulation of non-canonical NF-kappaB signal transduction / positive regulation of protein catabolic process / positive regulation of canonical Wnt signaling pathway / activation of cysteine-type endopeptidase activity involved in apoptotic process / azurophil granule lumen / KEAP1-NFE2L2 pathway / double-strand break repair / Ovarian tumor domain proteases / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / E3 ubiquitin ligases ubiquitinate target proteins / site of double-strand break / cellular response to heat /  Neddylation / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / Neddylation / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process /  protein phosphatase binding / secretory granule lumen / regulation of apoptotic process / ficolin-1-rich granule lumen / RNA polymerase II-specific DNA-binding transcription factor binding / Attachment and Entry / protein ubiquitination / protein domain specific binding / intracellular membrane-bounded organelle / protein phosphatase binding / secretory granule lumen / regulation of apoptotic process / ficolin-1-rich granule lumen / RNA polymerase II-specific DNA-binding transcription factor binding / Attachment and Entry / protein ubiquitination / protein domain specific binding / intracellular membrane-bounded organelle /  DNA repair / glutamatergic synapse / DNA repair / glutamatergic synapse /  lipid binding / DNA damage response / lipid binding / DNA damage response /  ubiquitin protein ligase binding / Neutrophil degranulation / endoplasmic reticulum membrane / perinuclear region of cytoplasm / ubiquitin protein ligase binding / Neutrophil degranulation / endoplasmic reticulum membrane / perinuclear region of cytoplasm /  endoplasmic reticulum / endoplasmic reticulum /  ATP hydrolysis activity ATP hydrolysis activitySimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.402 Å MOLECULAR REPLACEMENT / Resolution: 2.402 Å | ||||||
 Authors Authors | Jiang, T. / Li, Z. / Wang, Y. / Xu, M. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: Biochem. Biophys. Res. Commun. / Year: 2017 Journal: Biochem. Biophys. Res. Commun. / Year: 2017Title: Crystal structures of the UBX domain of human UBXD7 and its complex with p97 ATPase Authors: Li, Z.H. / Wang, Y. / Xu, M. / Jiang, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5x4l.cif.gz 5x4l.cif.gz | 211.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5x4l.ent.gz pdb5x4l.ent.gz | 170.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5x4l.json.gz 5x4l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/x4/5x4l https://data.pdbj.org/pub/pdb/validation_reports/x4/5x4l ftp://data.pdbj.org/pub/pdb/validation_reports/x4/5x4l ftp://data.pdbj.org/pub/pdb/validation_reports/x4/5x4l | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 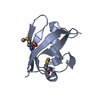 5x3pC  3qq7S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 20212.734 Da / Num. of mol.: 2 / Fragment: UNP residues 23-196 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: VCP / Production host: Homo sapiens (human) / Gene: VCP / Production host:   Escherichia coli (E. coli) / References: UniProt: P55072, Escherichia coli (E. coli) / References: UniProt: P55072,  vesicle-fusing ATPase vesicle-fusing ATPase#2: Protein | Mass: 9633.721 Da / Num. of mol.: 2 / Fragment: UNP residues 410-489 / Mutation: L472M Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: UBXN7, KIAA0794, UBXD7 / Production host: Homo sapiens (human) / Gene: UBXN7, KIAA0794, UBXD7 / Production host:   Escherichia coli (E. coli) / References: UniProt: O94888 Escherichia coli (E. coli) / References: UniProt: O94888#3: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 50.88 % |
|---|---|
Crystal grow | Temperature: 289 K / Method: vapor diffusion Details: 0.2M Calcium acetate hydrate pH7.5, 20% v/v PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL18U1 / Wavelength: 0.9777 Å / Beamline: BL18U1 / Wavelength: 0.9777 Å |
| Detector | Type: DECTRIS PILATUS3 S 6M / Detector: PIXEL / Date: Oct 23, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9777 Å / Relative weight: 1 : 0.9777 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→45.92 Å / Num. obs: 23521 / % possible obs: 100 % / Redundancy: 6.9 % / Rmerge(I) obs: 0.109 / Rpim(I) all: 0.031 / Net I/σ(I): 27 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3QQ7 Resolution: 2.402→45.92 Å / SU ML: 0.23 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 21.86 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.402→45.92 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj















