[English] 日本語
 Yorodumi
Yorodumi- PDB-5ou7: Crystal structure of the Glycoprotein VI loop truncation mutant P... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ou7 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the Glycoprotein VI loop truncation mutant PAVS-PAPYKN | ||||||
 Components Components | Platelet glycoprotein VI | ||||||
 Keywords Keywords |  BLOOD CLOTTING / BLOOD CLOTTING /  Platelet / Platelet /  glycoprotein / glycoprotein /  GPVI / collagen-binding / GPVI / collagen-binding /  platelet activation platelet activation | ||||||
| Function / homology |  Function and homology information Function and homology informationtetraspanin-enriched microdomain / collagen-activated signaling pathway / Platelet Adhesion to exposed collagen / positive regulation of platelet aggregation /  enzyme-linked receptor protein signaling pathway / GPVI-mediated activation cascade / enzyme-linked receptor protein signaling pathway / GPVI-mediated activation cascade /  collagen binding / collagen binding /  protein tyrosine kinase binding / Cell surface interactions at the vascular wall / protein tyrosine kinase binding / Cell surface interactions at the vascular wall /  platelet activation ...tetraspanin-enriched microdomain / collagen-activated signaling pathway / Platelet Adhesion to exposed collagen / positive regulation of platelet aggregation / platelet activation ...tetraspanin-enriched microdomain / collagen-activated signaling pathway / Platelet Adhesion to exposed collagen / positive regulation of platelet aggregation /  enzyme-linked receptor protein signaling pathway / GPVI-mediated activation cascade / enzyme-linked receptor protein signaling pathway / GPVI-mediated activation cascade /  collagen binding / collagen binding /  protein tyrosine kinase binding / Cell surface interactions at the vascular wall / protein tyrosine kinase binding / Cell surface interactions at the vascular wall /  platelet activation / transmembrane signaling receptor activity / platelet activation / transmembrane signaling receptor activity /  signaling receptor activity / signaling receptor activity /  membrane raft / membrane raft /  cell surface / extracellular exosome / cell surface / extracellular exosome /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å | ||||||
 Authors Authors | Feitsma, L.J. / Huizinga, E.G. | ||||||
| Funding support |  Netherlands, 1items Netherlands, 1items
| ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structural insights into collagen-binding by platelet receptor Glycoprotein VI Authors: Feitsma, L.J. / Brondijk, T.H.C. / Jarvis, G. / Hagemans, D. / Bihan, D. / Jerah, N. / Versteeg, M. / Farndale, R.W. / Huizinga, E.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ou7.cif.gz 5ou7.cif.gz | 295.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ou7.ent.gz pdb5ou7.ent.gz | 241.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ou7.json.gz 5ou7.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ou/5ou7 https://data.pdbj.org/pub/pdb/validation_reports/ou/5ou7 ftp://data.pdbj.org/pub/pdb/validation_reports/ou/5ou7 ftp://data.pdbj.org/pub/pdb/validation_reports/ou/5ou7 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5ou9C  2gi7S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 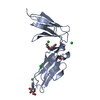
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 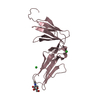
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Beg auth comp-ID: SER / Beg label comp-ID: SER / Refine code: 2
NCS ensembles :
NCS oper:
|
- Components
Components
-Protein / Sugars , 2 types, 8 molecules ABCD

| #1: Protein | Mass: 19719.215 Da / Num. of mol.: 4 / Mutation: -102-105 -131-136 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: GP6 / Cell line (production host): HEK293-EBNA1-S / Production host: Homo sapiens (human) / Gene: GP6 / Cell line (production host): HEK293-EBNA1-S / Production host:   Homo sapiens (human) / References: UniProt: Q9HCN6 Homo sapiens (human) / References: UniProt: Q9HCN6#5: Sugar | ChemComp-NAG /  N-Acetylglucosamine N-Acetylglucosamine |
|---|
-Non-polymers , 4 types, 362 molecules 

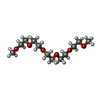




| #2: Chemical | ChemComp-CL /  Chloride Chloride#3: Chemical |  Phosphate Phosphate#4: Chemical |  Polyethylene glycol Polyethylene glycol#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 50.87 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 4 Details: 0.1 M phosphate-citrate buffer pH 4.0 40% (v/v) PEG-300 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 0.99999 Å / Beamline: X06SA / Wavelength: 0.99999 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Aug 22, 2013 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.99999 Å / Relative weight: 1 : 0.99999 Å / Relative weight: 1 |
| Reflection | Resolution: 1.9→113.81 Å / Num. obs: 58873 / % possible obs: 95.1 % / Redundancy: 2.1 % / Biso Wilson estimate: 25.6 Å2 / CC1/2: 0.99 / Rmerge(I) obs: 0.081 / Net I/σ(I): 5 |
| Reflection shell | Resolution: 1.9→1.94 Å / Redundancy: 2.1 % / Rmerge(I) obs: 1.08 / Mean I/σ(I) obs: 1.1 / Num. unique obs: 3656 / CC1/2: 0.62 / % possible all: 93.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2GI7 Resolution: 1.9→113.81 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.939 / SU B: 10.313 / SU ML: 0.143 / Cross valid method: THROUGHOUT / ESU R: 0.192 / ESU R Free: 0.169 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 33.189 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.9→113.81 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj









