+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ja9 | ||||||
|---|---|---|---|---|---|---|---|
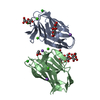
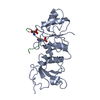
| Title | Crystal structure of the HigB2 toxin in complex with Nb6 | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  TOXIN / TOXIN /  toxin-antitoxin system toxin-antitoxin system | ||||||
| Function / homology | Toxin HigB-2 / RelE toxin of RelE / RelB toxin-antitoxin system /  Immunoglobulins / Immunoglobulins /  Immunoglobulin-like / Immunoglobulin-like /  Sandwich / Mainly Beta / Toxin HigB-2 Sandwich / Mainly Beta / Toxin HigB-2 Function and homology information Function and homology information | ||||||
| Biological species |   Vibrio cholerae serotype O1 (bacteria) Vibrio cholerae serotype O1 (bacteria)  Lama glama (llama) Lama glama (llama) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.849 Å MOLECULAR REPLACEMENT / Resolution: 1.849 Å | ||||||
 Authors Authors | Hadzi, S. / Loris, R. | ||||||
 Citation Citation |  Journal: Nucleic Acids Res. / Year: 2017 Journal: Nucleic Acids Res. / Year: 2017Title: Ribosome-dependent Vibrio cholerae mRNAse HigB2 is regulated by a beta-strand sliding mechanism. Authors: Hadzi, S. / Garcia-Pino, A. / Haesaerts, S. / Jurenas, D. / Gerdes, K. / Lah, J. / Loris, R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ja9.cif.gz 5ja9.cif.gz | 195.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ja9.ent.gz pdb5ja9.ent.gz | 163 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ja9.json.gz 5ja9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ja/5ja9 https://data.pdbj.org/pub/pdb/validation_reports/ja/5ja9 ftp://data.pdbj.org/pub/pdb/validation_reports/ja/5ja9 ftp://data.pdbj.org/pub/pdb/validation_reports/ja/5ja9 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
NCS ensembles :
|
- Components
Components
| #1: Protein | Mass: 13025.962 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Vibrio cholerae serotype O1 (strain ATCC 39315 / El Tor Inaba N16961) (bacteria) Vibrio cholerae serotype O1 (strain ATCC 39315 / El Tor Inaba N16961) (bacteria)Strain: ATCC 39315 / El Tor Inaba N16961 / Gene: higB-2, VC_A0468 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q9KMA6 Escherichia coli (E. coli) / References: UniProt: Q9KMA6#2: Antibody |  Single-domain antibody Single-domain antibodyMass: 13528.946 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Lama glama (llama) / Production host: Lama glama (llama) / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)#3: Chemical | ChemComp-SO4 /  Sulfate Sulfate#4: Chemical | ChemComp-EDO / |  Ethylene glycol Ethylene glycol#5: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.25 Å3/Da / Density % sol: 45.4 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop Details: 0.1M Hepes pH 7.5, 10% (w/v) PEG8000, 8% (w/v) Ethylene glycol |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.97903 Å / Beamline: PROXIMA 1 / Wavelength: 0.97903 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: PSI PILATUS 6M / Detector: PIXEL / Date: Jun 20, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.849→49.404 Å / Num. obs: 38530 / % possible obs: 95.8 % / Observed criterion σ(I): -3 / Redundancy: 3.44 % / Biso Wilson estimate: 26.46 Å2 / CC1/2: 0.999 / Rmerge(I) obs: 0.075 / Net I/σ(I): 10.27 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 1.849→44.35 Å / SU ML: 0.24 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 31.11 MOLECULAR REPLACEMENT / Resolution: 1.849→44.35 Å / SU ML: 0.24 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 31.11
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 156.8 Å2 / Biso mean: 53.2997 Å2 / Biso min: 17.04 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.849→44.35 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 14
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj






