[English] 日本語
 Yorodumi
Yorodumi- PDB-5h9o: Complex of Murine endoplasmic reticulum alpha-glucosidase II with... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5h9o | ||||||
|---|---|---|---|---|---|---|---|
| Title | Complex of Murine endoplasmic reticulum alpha-glucosidase II with D-Glucose | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  HYDROLASE / Enzyme Glycosyl hydrolase GH31 Quality control exoglycosidase HYDROLASE / Enzyme Glycosyl hydrolase GH31 Quality control exoglycosidase | ||||||
| Function / homology |  Function and homology information Function and homology informationmannosyl-oligosaccharide alpha-1,3-glucosidase /  glucan 1,3-alpha-glucosidase activity / glucan 1,3-alpha-glucosidase activity /  glucosidase II complex / glucosidase II complex /  alpha-glucosidase activity / alpha-glucosidase activity /  glucosidase activity / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / glucosidase activity / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) /  Post-translational protein phosphorylation / N-glycan processing / liver development / Post-translational protein phosphorylation / N-glycan processing / liver development /  melanosome ...mannosyl-oligosaccharide alpha-1,3-glucosidase / melanosome ...mannosyl-oligosaccharide alpha-1,3-glucosidase /  glucan 1,3-alpha-glucosidase activity / glucan 1,3-alpha-glucosidase activity /  glucosidase II complex / glucosidase II complex /  alpha-glucosidase activity / alpha-glucosidase activity /  glucosidase activity / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / glucosidase activity / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) /  Post-translational protein phosphorylation / N-glycan processing / liver development / Post-translational protein phosphorylation / N-glycan processing / liver development /  melanosome / negative regulation of neuron projection development / melanosome / negative regulation of neuron projection development /  carbohydrate binding / in utero embryonic development / carbohydrate metabolic process / intracellular membrane-bounded organelle / carbohydrate binding / in utero embryonic development / carbohydrate metabolic process / intracellular membrane-bounded organelle /  calcium ion binding / protein-containing complex binding / calcium ion binding / protein-containing complex binding /  Golgi apparatus / Golgi apparatus /  endoplasmic reticulum / endoplasmic reticulum /  RNA binding RNA bindingSimilarity search - Function | ||||||
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.37 Å MOLECULAR REPLACEMENT / Resolution: 2.37 Å | ||||||
 Authors Authors | Caputo, A.T. / Roversi, P. / Alonzi, D.S. / Kiappes, J.L. / Zitzmann, N. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2016 Journal: Proc.Natl.Acad.Sci.USA / Year: 2016Title: Structures of mammalian ER alpha-glucosidase II capture the binding modes of broad-spectrum iminosugar antivirals. Authors: Caputo, A.T. / Alonzi, D.S. / Marti, L. / Reca, I.B. / Kiappes, J.L. / Struwe, W.B. / Cross, A. / Basu, S. / Lowe, E.D. / Darlot, B. / Santino, A. / Roversi, P. / Zitzmann, N. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5h9o.cif.gz 5h9o.cif.gz | 725.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5h9o.ent.gz pdb5h9o.ent.gz | 611.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5h9o.json.gz 5h9o.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h9/5h9o https://data.pdbj.org/pub/pdb/validation_reports/h9/5h9o ftp://data.pdbj.org/pub/pdb/validation_reports/h9/5h9o ftp://data.pdbj.org/pub/pdb/validation_reports/h9/5h9o | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5f0eC  5hjoC  5hjrC  5iedC  5ieeC  5iefC  5iegC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 2 types, 4 molecules ACBD
| #1: Protein | Mass: 97894.625 Da / Num. of mol.: 2 / Fragment: UNP Residues 33-944 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Gene: Ganab, G2an, Kiaa0088 / Plasmid: pHLsec / Cell line (production host): HEK293-F / Organ (production host): Kidney / Production host: Mus musculus (house mouse) / Gene: Ganab, G2an, Kiaa0088 / Plasmid: pHLsec / Cell line (production host): HEK293-F / Organ (production host): Kidney / Production host:   Homo sapiens (human) / References: UniProt: Q8BHN3, EC: 3.2.1.84 Homo sapiens (human) / References: UniProt: Q8BHN3, EC: 3.2.1.84#2: Protein |  Glucosidases / 80K-H protein / Glucosidase II subunit beta / Protein kinase C substrate 60.1 kDa protein heavy chain / PKCSH Glucosidases / 80K-H protein / Glucosidase II subunit beta / Protein kinase C substrate 60.1 kDa protein heavy chain / PKCSHMass: 9568.298 Da / Num. of mol.: 2 / Fragment: UNP residues 30-117 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Gene: Prkcsh / Plasmid: pHLsec / Cell line (production host): HEK293-F / Production host: Mus musculus (house mouse) / Gene: Prkcsh / Plasmid: pHLsec / Cell line (production host): HEK293-F / Production host:   Homo sapiens (human) / References: UniProt: O08795 Homo sapiens (human) / References: UniProt: O08795 |
|---|
-Sugars , 2 types, 6 molecules 
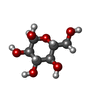

| #3: Sugar | ChemComp-NAG /  N-Acetylglucosamine N-Acetylglucosamine#9: Sugar |  Glucose Glucose |
|---|
-Non-polymers , 8 types, 476 molecules 





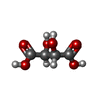








| #4: Chemical | ChemComp-EDO /  Ethylene glycol Ethylene glycol#5: Chemical | ChemComp-FMT /  Formic acid Formic acid#6: Chemical | ChemComp-PG4 /  Polyethylene glycol Polyethylene glycol#7: Chemical | ChemComp-P6G /  Polyethylene glycol Polyethylene glycol#8: Chemical | ChemComp-OXM / |  Oxamic acid Oxamic acid#10: Chemical | ChemComp-CA / #11: Chemical | ChemComp-TAR / |  Tartaric acid Tartaric acid#12: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.3 Å3/Da / Density % sol: 46.61 % |
|---|---|
Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 6.25 Details: 32 % Morpheus ethylene glycol/PEG 8000 mix, 0.05 M Morpheus carboxylic acids mix, 0.1 M Morpheus buffer system 1 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.9795 Å / Beamline: I04 / Wavelength: 0.9795 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Sep 27, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9795 Å / Relative weight: 1 : 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 2.37→87.88 Å / Num. obs: 89708 / % possible obs: 99.8 % / Redundancy: 5.5 % / Biso Wilson estimate: 33.37 Å2 / Rsym value: 0.24 / Net I/σ(I): 5.1 |
| Reflection shell | Resolution: 2.37→2.43 Å / Redundancy: 5.4 % / Mean I/σ(I) obs: 1.2 / Num. unique all: 6578 / Rsym value: 1.53 / % possible all: 99.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 2.37→87.66 Å / Cor.coef. Fo:Fc: 0.905 / Cor.coef. Fo:Fc free: 0.882 / Rfactor Rfree error: 0 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.414 / SU Rfree Blow DPI: 0.25 MOLECULAR REPLACEMENT / Resolution: 2.37→87.66 Å / Cor.coef. Fo:Fc: 0.905 / Cor.coef. Fo:Fc free: 0.882 / Rfactor Rfree error: 0 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.414 / SU Rfree Blow DPI: 0.25
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 33.8 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.37 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.37→87.66 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.37→2.43 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


















