[English] 日本語
 Yorodumi
Yorodumi- PDB-5aha: Disubstituted bis-THF moieties as new P2 ligands in non-peptidal ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5aha | ||||||
|---|---|---|---|---|---|---|---|
| Title | Disubstituted bis-THF moieties as new P2 ligands in non-peptidal HIV- 1 Protease Inhibitors (II) | ||||||
 Components Components | PROTEASE | ||||||
 Keywords Keywords |  HYDROLASE / HYDROLASE /  INHIBITOR / INHIBITOR /  RATIONAL DRUG DESIGN / BIS-THF BIS-DIOL RATIONAL DRUG DESIGN / BIS-THF BIS-DIOL | ||||||
| Function / homology |  Function and homology information Function and homology information: / : /  HIV-1 retropepsin / HIV-1 retropepsin /  retroviral ribonuclease H / retroviral ribonuclease H /  exoribonuclease H / exoribonuclease H /  exoribonuclease H activity / host multivesicular body / DNA integration / exoribonuclease H activity / host multivesicular body / DNA integration /  RNA-directed DNA polymerase / viral penetration into host nucleus ...: / : / RNA-directed DNA polymerase / viral penetration into host nucleus ...: / : /  HIV-1 retropepsin / HIV-1 retropepsin /  retroviral ribonuclease H / retroviral ribonuclease H /  exoribonuclease H / exoribonuclease H /  exoribonuclease H activity / host multivesicular body / DNA integration / exoribonuclease H activity / host multivesicular body / DNA integration /  RNA-directed DNA polymerase / viral penetration into host nucleus / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / viral penetration into host nucleus / viral genome integration into host DNA / establishment of integrated proviral latency /  RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity /  Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / viral nucleocapsid / DNA recombination / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / viral nucleocapsid / DNA recombination /  Hydrolases; Acting on ester bonds / Hydrolases; Acting on ester bonds /  DNA-directed DNA polymerase / aspartic-type endopeptidase activity / DNA-directed DNA polymerase / aspartic-type endopeptidase activity /  DNA-directed DNA polymerase activity / symbiont entry into host cell / symbiont-mediated suppression of host gene expression / DNA-directed DNA polymerase activity / symbiont entry into host cell / symbiont-mediated suppression of host gene expression /  lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity /  proteolysis / proteolysis /  DNA binding / DNA binding /  RNA binding / zinc ion binding / RNA binding / zinc ion binding /  membrane membraneSimilarity search - Function | ||||||
| Biological species |    HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.35 Å MOLECULAR REPLACEMENT / Resolution: 1.35 Å | ||||||
 Authors Authors | Hohlfeld, K. / Wegner, J.K. / Kesteleyn, B. / Linclau, B. / Unge, J. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2015 Journal: J.Med.Chem. / Year: 2015Title: Disubstituted Bis-Thf Moieties as New P2 Ligands in Non-Peptidal HIV-1 Protease Inhibitors (II). Authors: Hohlfeld, K. / Wegner, J. / Kesteleyn, B. / Linclau, B. / Unge, J. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AB" IN EACH CHAIN ON SHEET RECORDS BELOW ... SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AB" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 6-STRANDED BARREL THIS IS REPRESENTED BY A 7-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "BA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 6-STRANDED BARREL THIS IS REPRESENTED BY A 7-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5aha.cif.gz 5aha.cif.gz | 60.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5aha.ent.gz pdb5aha.ent.gz | 44.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5aha.json.gz 5aha.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ah/5aha https://data.pdbj.org/pub/pdb/validation_reports/ah/5aha ftp://data.pdbj.org/pub/pdb/validation_reports/ah/5aha ftp://data.pdbj.org/pub/pdb/validation_reports/ah/5aha | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5agzC  5ah6C  5ah7C  5ah8C  5ah9C  5ahbC  5ahcC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 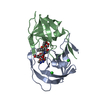
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein |  Mass: 10775.659 Da / Num. of mol.: 2 / Fragment: ASPARTYL PROTEASE, RESIDUES 501-599 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)   HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 (Z2/CDC-Z34 ISOLATE) HUMAN IMMUNODEFICIENCY VIRUS TYPE 1 (Z2/CDC-Z34 ISOLATE)Strain: 99HHP1 (D10) / Plasmid: PEXP5 / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(AI) / References: UniProt: P03366, ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(AI) / References: UniProt: P03366,  HIV-1 retropepsin HIV-1 retropepsin#2: Chemical |  Chloride Chloride#3: Chemical | ChemComp-ZLP / ( | #4: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.7 Å3/Da / Density % sol: 54.49 % / Description: NONE |
|---|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  MAX II MAX II  / Beamline: I911-3 / Wavelength: 1 / Beamline: I911-3 / Wavelength: 1 |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Apr 17, 2012 / Details: MIRRORS |
| Radiation | Monochromator: SI / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.35→58.4 Å / Num. obs: 52307 / % possible obs: 100 % / Observed criterion σ(I): 4 / Redundancy: 6.2 % / Rmerge(I) obs: 0.08 / Net I/σ(I): 5.8 |
| Reflection shell | Resolution: 1.35→1.42 Å / Redundancy: 5.2 % / Rmerge(I) obs: 0.39 / Mean I/σ(I) obs: 1.7 / % possible all: 99.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: NON-PUBLISHED Resolution: 1.35→48.29 Å / Cor.coef. Fo:Fc: 0.945 / Cor.coef. Fo:Fc free: 0.941 / SU B: 0.878 / SU ML: 0.037 / Cross valid method: THROUGHOUT / ESU R: 0.061 / ESU R Free: 0.06 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 15.778 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.35→48.29 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


















 PDBj
PDBj






