[English] 日本語
 Yorodumi
Yorodumi- PDB-4xjs: Human CD38 complexed with inhibitor 1 [6-fluoro-2-methyl-4-[(2,3,... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4xjs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human CD38 complexed with inhibitor 1 [6-fluoro-2-methyl-4-[(2,3,6-trichlorobenzyl)amino]quinoline-8-carboxamide] | |||||||||
 Components Components | ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1 | |||||||||
 Keywords Keywords |  Hydrolase / Hydrolase /  Transferase / Transferase /  CD38 CD38 | |||||||||
| Function / homology |  Function and homology information Function and homology information2'-phospho-ADP-ribosyl cyclase/2'-phospho-cyclic-ADP-ribose transferase / phosphorus-oxygen lyase activity / artery smooth muscle contraction /  Nicotinate metabolism / Nicotinate metabolism /  NAD+ nucleosidase activity / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NADP+ nucleosidase activity / NAD metabolic process / NAD+ nucleotidase, cyclic ADP-ribose generating / negative regulation of bone resorption ...2'-phospho-ADP-ribosyl cyclase/2'-phospho-cyclic-ADP-ribose transferase / phosphorus-oxygen lyase activity / artery smooth muscle contraction / NAD+ nucleosidase activity / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NADP+ nucleosidase activity / NAD metabolic process / NAD+ nucleotidase, cyclic ADP-ribose generating / negative regulation of bone resorption ...2'-phospho-ADP-ribosyl cyclase/2'-phospho-cyclic-ADP-ribose transferase / phosphorus-oxygen lyase activity / artery smooth muscle contraction /  Nicotinate metabolism / Nicotinate metabolism /  NAD+ nucleosidase activity / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NADP+ nucleosidase activity / NAD metabolic process / NAD+ nucleotidase, cyclic ADP-ribose generating / negative regulation of bone resorption / response to hydroperoxide / long-term synaptic depression / NAD+ nucleosidase activity / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NADP+ nucleosidase activity / NAD metabolic process / NAD+ nucleotidase, cyclic ADP-ribose generating / negative regulation of bone resorption / response to hydroperoxide / long-term synaptic depression /  Hydrolases; Glycosylases; Hydrolysing N-glycosyl compounds / B cell proliferation / response to retinoic acid / positive regulation of B cell proliferation / positive regulation of vasoconstriction / response to interleukin-1 / response to progesterone / female pregnancy / apoptotic signaling pathway / B cell receptor signaling pathway / positive regulation of insulin secretion / response to estradiol / negative regulation of neuron projection development / positive regulation of cytosolic calcium ion concentration / Hydrolases; Glycosylases; Hydrolysing N-glycosyl compounds / B cell proliferation / response to retinoic acid / positive regulation of B cell proliferation / positive regulation of vasoconstriction / response to interleukin-1 / response to progesterone / female pregnancy / apoptotic signaling pathway / B cell receptor signaling pathway / positive regulation of insulin secretion / response to estradiol / negative regulation of neuron projection development / positive regulation of cytosolic calcium ion concentration /  transferase activity / positive regulation of cell growth / basolateral plasma membrane / response to hypoxia / response to xenobiotic stimulus / negative regulation of DNA-templated transcription / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / transferase activity / positive regulation of cell growth / basolateral plasma membrane / response to hypoxia / response to xenobiotic stimulus / negative regulation of DNA-templated transcription / negative regulation of apoptotic process / positive regulation of DNA-templated transcription /  cell surface / cell surface /  signal transduction / extracellular exosome / signal transduction / extracellular exosome /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.8 Å MOLECULAR REPLACEMENT / Resolution: 2.8 Å | |||||||||
 Authors Authors | Shewchuk, L.M. / Deaton, D. / Stewart, E. | |||||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2015 Journal: J.Med.Chem. / Year: 2015Title: Discovery of 4-Amino-8-quinoline Carboxamides as Novel, Submicromolar Inhibitors of NAD-Hydrolyzing Enzyme CD38. Authors: Becherer, J.D. / Boros, E.E. / Carpenter, T.Y. / Cowan, D.J. / Deaton, D.N. / Haffner, C.D. / Jeune, M.R. / Kaldor, I.W. / Poole, J.C. / Preugschat, F. / Rheault, T.R. / Schulte, C.A. / ...Authors: Becherer, J.D. / Boros, E.E. / Carpenter, T.Y. / Cowan, D.J. / Deaton, D.N. / Haffner, C.D. / Jeune, M.R. / Kaldor, I.W. / Poole, J.C. / Preugschat, F. / Rheault, T.R. / Schulte, C.A. / Shearer, B.G. / Shearer, T.W. / Shewchuk, L.M. / Smalley, T.L. / Stewart, E.L. / Stuart, J.D. / Ulrich, J.C. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4xjs.cif.gz 4xjs.cif.gz | 114.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4xjs.ent.gz pdb4xjs.ent.gz | 85.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4xjs.json.gz 4xjs.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xj/4xjs https://data.pdbj.org/pub/pdb/validation_reports/xj/4xjs ftp://data.pdbj.org/pub/pdb/validation_reports/xj/4xjs ftp://data.pdbj.org/pub/pdb/validation_reports/xj/4xjs | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4xjtC  1yh3S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 30318.303 Da / Num. of mol.: 1 / Mutation: N100D, N164A, N209D, N219D Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: CD38 / Production host: Homo sapiens (human) / Gene: CD38 / Production host:   Pichia (fungus) Pichia (fungus)References: UniProt: P28907, ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase, 2'-phospho-ADP-ribosyl cyclase/2'-phospho-cyclic-ADP-ribose transferase |
|---|---|
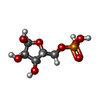
| #2: Chemical | ChemComp-733 / |
| #3: Sugar | ChemComp-HSX / |
| #4: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.95 Å3/Da / Density % sol: 36.8 % |
|---|---|
Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: Protein concentration was 7mgs/ml. Crystals were grown from 23% PEG3350, 0.1mM BisTrisPropane at 22 deg C (2+2uL drops over a 500uL well). Crystals were soaked with 5mM inhibitor for 24 ...Details: Protein concentration was 7mgs/ml. Crystals were grown from 23% PEG3350, 0.1mM BisTrisPropane at 22 deg C (2+2uL drops over a 500uL well). Crystals were soaked with 5mM inhibitor for 24 hours prior to data collection. Crystals were flash frozen in PFO. |
-Data collection
| Diffraction | Mean temperature: 93 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.54 Å ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.54 Å |
| Detector | Type: RIGAKU SATURN 944+ / Detector: CCD / Date: Jan 25, 2011 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.54 Å / Relative weight: 1 : 1.54 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→34.8 Å / Num. obs: 5790 / % possible obs: 98 % / Redundancy: 6.6 % / Rmerge(I) obs: 0.09 / Net I/σ(I): 29 |
| Reflection shell | Resolution: 2.8→2.87 Å / Redundancy: 7 % / Rmerge(I) obs: 0.59 / Mean I/σ(I) obs: 4.8 / % possible all: 99.5 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1YH3 Resolution: 2.8→34.8 Å / Cor.coef. Fo:Fc: 0.945 / Cor.coef. Fo:Fc free: 0.896 / SU B: 36.526 / SU ML: 0.351 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R Free: 0.439 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : WITH TLS ADDED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 115.38 Å2 / Biso mean: 53.927 Å2 / Biso min: 14.79 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.8→34.8 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.8→2.872 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Details: Chain A catalytic domain / Origin x: -7.141 Å / Origin y: -14.844 Å / Origin z: 15.142 Å
|
 Movie
Movie Controller
Controller












 PDBj
PDBj