[English] 日本語
 Yorodumi
Yorodumi- PDB-4w9n: Enoyl-acyl carrier protein-reductase domain from human fatty acid... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4w9n | ||||||
|---|---|---|---|---|---|---|---|
| Title | Enoyl-acyl carrier protein-reductase domain from human fatty acid synthase complexed with triclosan | ||||||
 Components Components | (Enoyl-[acyl-carrier-protein] ...) x 2 | ||||||
 Keywords Keywords |  OXIDOREDUCTASE / OXIDOREDUCTASE /  fatty acid synthase / fatty acid synthase /  fatty acid metabolism / NADPH-dependent / enoyl reductase fatty acid metabolism / NADPH-dependent / enoyl reductase | ||||||
| Function / homology |  Function and homology information Function and homology informationfatty-acid synthase system / (3R)-3-hydroxybutanoyl-[acyl-carrier-protein] hydratase activity / fatty acyl-[ACP] hydrolase activity / ether lipid biosynthetic process / (3R)-3-hydroxyoctanoyl-[acyl-carrier-protein] dehydratase activity / Vitamin B5 (pantothenate) metabolism / neutrophil differentiation / enoyl-[acyl-carrier-protein] reductase (NADPH, Re-specific) / (3R)-3-hydroxydecanoyl-[acyl-carrier-protein] dehydratase activity / glandular epithelial cell development ...fatty-acid synthase system / (3R)-3-hydroxybutanoyl-[acyl-carrier-protein] hydratase activity / fatty acyl-[ACP] hydrolase activity / ether lipid biosynthetic process / (3R)-3-hydroxyoctanoyl-[acyl-carrier-protein] dehydratase activity / Vitamin B5 (pantothenate) metabolism / neutrophil differentiation / enoyl-[acyl-carrier-protein] reductase (NADPH, Re-specific) / (3R)-3-hydroxydecanoyl-[acyl-carrier-protein] dehydratase activity / glandular epithelial cell development / : /  glycogen granule / establishment of endothelial intestinal barrier / [acyl-carrier-protein] S-acetyltransferase / [acyl-carrier-protein] S-acetyltransferase activity / Fatty acyl-CoA biosynthesis / oleoyl-[acyl-carrier-protein] hydrolase / (3R)-3-hydroxypalmitoyl-[acyl-carrier-protein] dehydratase activity / modulation by host of viral process / (3R)-3-hydroxymyristoyl-[acyl-carrier-protein] dehydratase activity / ChREBP activates metabolic gene expression / [acyl-carrier-protein] S-malonyltransferase / [acyl-carrier-protein] S-malonyltransferase activity / 3-hydroxyacyl-[acyl-carrier-protein] dehydratase / beta-ketoacyl-[acyl-carrier-protein] synthase I / NR1H2 & NR1H3 regulate gene expression linked to lipogenesis / glycogen granule / establishment of endothelial intestinal barrier / [acyl-carrier-protein] S-acetyltransferase / [acyl-carrier-protein] S-acetyltransferase activity / Fatty acyl-CoA biosynthesis / oleoyl-[acyl-carrier-protein] hydrolase / (3R)-3-hydroxypalmitoyl-[acyl-carrier-protein] dehydratase activity / modulation by host of viral process / (3R)-3-hydroxymyristoyl-[acyl-carrier-protein] dehydratase activity / ChREBP activates metabolic gene expression / [acyl-carrier-protein] S-malonyltransferase / [acyl-carrier-protein] S-malonyltransferase activity / 3-hydroxyacyl-[acyl-carrier-protein] dehydratase / beta-ketoacyl-[acyl-carrier-protein] synthase I / NR1H2 & NR1H3 regulate gene expression linked to lipogenesis /  mammary gland development / 3-oxoacyl-[acyl-carrier-protein] reductase / 3-oxoacyl-[acyl-carrier-protein] reductase (NADPH) activity / mammary gland development / 3-oxoacyl-[acyl-carrier-protein] reductase / 3-oxoacyl-[acyl-carrier-protein] reductase (NADPH) activity /  fatty acid synthase activity / fatty acid synthase activity /  phosphopantetheine binding / monocyte differentiation / 3-oxoacyl-[acyl-carrier-protein] synthase activity / cellular response to interleukin-4 / Activation of gene expression by SREBF (SREBP) / fatty acid metabolic process / fatty acid biosynthetic process / osteoblast differentiation / phosphopantetheine binding / monocyte differentiation / 3-oxoacyl-[acyl-carrier-protein] synthase activity / cellular response to interleukin-4 / Activation of gene expression by SREBF (SREBP) / fatty acid metabolic process / fatty acid biosynthetic process / osteoblast differentiation /  melanosome / melanosome /  cadherin binding / cadherin binding /  inflammatory response / inflammatory response /  Golgi apparatus / Golgi apparatus /  RNA binding / extracellular exosome / RNA binding / extracellular exosome /  membrane / membrane /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.84 Å molecular replacement / Resolution: 1.84 Å | ||||||
 Authors Authors | Sippel, K.H. / Vyas, N.K. / Sankaran, B. / Quiocho, F.A. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2014 Journal: J.Biol.Chem. / Year: 2014Title: Crystal structure of the human Fatty Acid synthase enoyl-acyl carrier protein-reductase domain complexed with triclosan reveals allosteric protein-protein interface inhibition. Authors: Sippel, K.H. / Vyas, N.K. / Zhang, W. / Sankaran, B. / Quiocho, F.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4w9n.cif.gz 4w9n.cif.gz | 699.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4w9n.ent.gz pdb4w9n.ent.gz | 584.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4w9n.json.gz 4w9n.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/w9/4w9n https://data.pdbj.org/pub/pdb/validation_reports/w9/4w9n ftp://data.pdbj.org/pub/pdb/validation_reports/w9/4w9n ftp://data.pdbj.org/pub/pdb/validation_reports/w9/4w9n | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4w82SC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 |
| ||||||||
| Unit cell |
|
- Components
Components
-Enoyl-[acyl-carrier-protein] ... , 2 types, 4 molecules ABDC
| #1: Protein | Mass: 36778.012 Da / Num. of mol.: 3 / Fragment: unp residues 1529-1867 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: FASN, FAS / Plasmid: ptyb21 / Production host: Homo sapiens (human) / Gene: FASN, FAS / Plasmid: ptyb21 / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: P49327, [acyl-carrier-protein] S-malonyltransferase #2: Protein | | Mass: 36836.047 Da / Num. of mol.: 1 / Fragment: unp residues 1529-1867 / Mutation: C1548(CSS) Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: FASN, FAS / Plasmid: ptyb21 / Production host: Homo sapiens (human) / Gene: FASN, FAS / Plasmid: ptyb21 / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: P49327, [acyl-carrier-protein] S-malonyltransferase |
|---|
-Non-polymers , 5 types, 538 molecules 


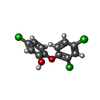





| #3: Chemical | ChemComp-IMD /  Imidazole Imidazole#4: Chemical | ChemComp-CL /  Chloride Chloride#5: Chemical | ChemComp-NA / #6: Chemical |  Triclosan Triclosan#7: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.38 Å3/Da / Density % sol: 48.36 % |
|---|---|
Crystal grow | Temperature: 296 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 20 mM NADP+ and 2 mM triclosan cocrystallized with 2.625 M sodium chloride, 100 mM imidazole pH 7.5, microseeded |
-Data collection
| Diffraction |
| |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.5418 Å | |||||||||||||||||||||||||||
| Detector |
| |||||||||||||||||||||||||||
| Radiation | Monochromator: VeriMax / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 | |||||||||||||||||||||||||||
| Reflection | Redundancy: 6.2 % / Number: 695699 / Rmerge(I) obs: 0.195 / D res high: 1.84 Å / D res low: 28.43 Å / Num. obs: 112996 / % possible obs: 95.9 / Rejects: 52 | |||||||||||||||||||||||||||
| Diffraction reflection shell |
| |||||||||||||||||||||||||||
| Reflection | Resolution: 1.84→28.43 Å / Num. obs: 112996 / % possible obs: 95.9 % / Redundancy: 6.2 % / Biso Wilson estimate: 24.65 Å2 / CC1/2: 0.995 / Rmerge(I) obs: 0.195 / Rpim(I) all: 0.08 / Net I/σ(I): 8.3 / Num. measured all: 695699 / Scaling rejects: 52 | |||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: 0
|
-Phasing
Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4W82 Resolution: 1.84→26.428 Å / SU ML: 0.21 / Cross valid method: FREE R-VALUE / σ(F): 1.92 / Phase error: 29.09 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 108.8 Å2 / Biso mean: 36.24 Å2 / Biso min: 13.61 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.84→26.428 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 30
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj














