[English] 日本語
 Yorodumi
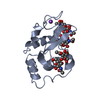
Yorodumi- PDB-4qf2: Crystal structure of human BAZ2A PHD zinc finger in the free form -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4qf2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human BAZ2A PHD zinc finger in the free form | ||||||
 Components Components | Bromodomain adjacent to zinc finger domain protein 2A | ||||||
 Keywords Keywords |  TRANSCRIPTION / Bromodomain adjacent to zinc finger domain protein 2A / Transcription termination factor I-interacting protein 5 / TTF-I-interacting protein 5 TRANSCRIPTION / Bromodomain adjacent to zinc finger domain protein 2A / Transcription termination factor I-interacting protein 5 / TTF-I-interacting protein 5 | ||||||
| Function / homology |  Function and homology information Function and homology informationNoRC complex / : / : / rDNA heterochromatin / rDNA heterochromatin formation / chromatin silencing complex / RNA polymerase I preinitiation complex assembly / : / negative regulation of transcription by RNA polymerase I /  : ...NoRC complex / : / : / rDNA heterochromatin / rDNA heterochromatin formation / chromatin silencing complex / RNA polymerase I preinitiation complex assembly / : / negative regulation of transcription by RNA polymerase I / : ...NoRC complex / : / : / rDNA heterochromatin / rDNA heterochromatin formation / chromatin silencing complex / RNA polymerase I preinitiation complex assembly / : / negative regulation of transcription by RNA polymerase I /  : / heterochromatin formation / : / heterochromatin formation /  nuclear receptor binding / lysine-acetylated histone binding / NoRC negatively regulates rRNA expression / nuclear receptor binding / lysine-acetylated histone binding / NoRC negatively regulates rRNA expression /  histone binding / nuclear speck / histone binding / nuclear speck /  chromatin remodeling / DNA-templated transcription / chromatin remodeling / DNA-templated transcription /  nucleolus / regulation of DNA-templated transcription / nucleolus / regulation of DNA-templated transcription /  DNA binding / DNA binding /  RNA binding / RNA binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 1.7 Å SAD / Resolution: 1.7 Å | ||||||
 Authors Authors | Tallant, C. / Overvoorde, L. / Van Molle, I. / Chirgadze, D.Y. / Ciulli, A. | ||||||
 Citation Citation |  Journal: Structure / Year: 2015 Journal: Structure / Year: 2015Title: Molecular basis of histone tail recognition by human TIP5 PHD finger and bromodomain of the chromatin remodeling complex NoRC. Authors: Cynthia Tallant / Erica Valentini / Oleg Fedorov / Lois Overvoorde / Fleur M Ferguson / Panagis Filippakopoulos / Dmitri I Svergun / Stefan Knapp / Alessio Ciulli /   Abstract: Binding of the chromatin remodeling complex NoRC to RNA complementary to the rDNA promoter mediates transcriptional repression. TIP5, the largest subunit of NoRC, is involved in recruitment to rDNA ...Binding of the chromatin remodeling complex NoRC to RNA complementary to the rDNA promoter mediates transcriptional repression. TIP5, the largest subunit of NoRC, is involved in recruitment to rDNA by interactions with promoter-bound TTF-I, pRNA, and acetylation of H4K16. TIP5 domains that recognize posttranslational modifications on histones are essential for recruitment of NoRC to chromatin, but how these reader modules recognize site-specific histone tails has remained elusive. Here, we report crystal structures of PHD zinc finger and bromodomains from human TIP5 and BAZ2B in free form and bound to H3 and/or H4 histones. PHD finger functions as an independent structural module in recognizing unmodified H3 histone tails, and the bromodomain prefers H3 and H4 acetylation marks followed by a key basic residue, KacXXR. Further low-resolution analyses of PHD-bromodomain modules provide molecular insights into their trans histone tail recognition, required for nucleosome recruitment and transcriptional repression of the NoRC complex. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4qf2.cif.gz 4qf2.cif.gz | 97.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4qf2.ent.gz pdb4qf2.ent.gz | 75.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4qf2.json.gz 4qf2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qf/4qf2 https://data.pdbj.org/pub/pdb/validation_reports/qf/4qf2 ftp://data.pdbj.org/pub/pdb/validation_reports/qf/4qf2 ftp://data.pdbj.org/pub/pdb/validation_reports/qf/4qf2 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4lz2C  4q6fC  4qbmC  4qc1C  4qc3C  4qf3C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 6597.694 Da / Num. of mol.: 4 / Fragment: unp residues 1673-1728 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: BAZ2A, KIAA0314, TIP5 / Plasmid: pET15b / Production host: Homo sapiens (human) / Gene: BAZ2A, KIAA0314, TIP5 / Plasmid: pET15b / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3)Rosetta / References: UniProt: Q9UIF9 Escherichia coli (E. coli) / Strain (production host): BL21(DE3)Rosetta / References: UniProt: Q9UIF9#2: Chemical | ChemComp-ZN / #3: Chemical |  Phosphate Phosphate#4: Chemical | ChemComp-GOL / |  Glycerol Glycerol#5: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.43 Å3/Da / Density % sol: 49.45 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8 Details: 2M Na/K phosphate, pH 8, VAPOR DIFFUSION, SITTING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I02 / Wavelength: 1.2822 Å / Beamline: I02 / Wavelength: 1.2822 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Oct 12, 2012 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 1.2822 Å / Relative weight: 1 : 1.2822 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.7→58.25 Å / Num. obs: 27299 / % possible obs: 100 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 14.8 % / Biso Wilson estimate: 19.4 Å2 / Rmerge(I) obs: 0.063 / Rsym value: 0.063 / Net I/σ(I): 24.9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / % possible all: 100
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  SAD SADStarting model: Arp/wArp autobuilding model Resolution: 1.7→58.25 Å / Cor.coef. Fo:Fc: 0.964 / Cor.coef. Fo:Fc free: 0.95 / SU B: 3.001 / SU ML: 0.059 / Cross valid method: THROUGHOUT / σ(I): 0 / ESU R: 0.094 / ESU R Free: 0.096 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 31.459 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.7→58.25 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.698→1.742 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj









