[English] 日本語
 Yorodumi
Yorodumi- PDB-4o9s: Crystal structure of Retinol-Binding Protein 4 (RBP4)in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4o9s | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
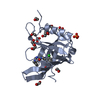
| Title | Crystal structure of Retinol-Binding Protein 4 (RBP4)in complex with a non-retinoid ligand | |||||||||
 Components Components | Retinol-binding protein 4 | |||||||||
 Keywords Keywords |  PROTEIN BINDING / PROTEIN BINDING /  retinol binding / Disease mutation / Retinol-binding / retinol binding / Disease mutation / Retinol-binding /  Secreted / Secreted /  Sensory transduction / Transport / Vision / Vitamin A / Sensory transduction / Transport / Vision / Vitamin A /  TRANSPORT PROTEIN TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationRetinoid metabolism disease events / urinary bladder development / embryonic retina morphogenesis in camera-type eye / retinol transport / female genitalia morphogenesis / retinol transmembrane transporter activity / embryonic organ morphogenesis / maintenance of gastrointestinal epithelium / embryonic skeletal system development / negative regulation of cardiac muscle cell proliferation ...Retinoid metabolism disease events / urinary bladder development / embryonic retina morphogenesis in camera-type eye / retinol transport / female genitalia morphogenesis / retinol transmembrane transporter activity / embryonic organ morphogenesis / maintenance of gastrointestinal epithelium / embryonic skeletal system development / negative regulation of cardiac muscle cell proliferation /  eye development / heart trabecula formation / cardiac muscle tissue development / eye development / heart trabecula formation / cardiac muscle tissue development /  retinal binding / retinol metabolic process / retinal binding / retinol metabolic process /  retinol binding / Retinoid cycle disease events / The canonical retinoid cycle in rods (twilight vision) / uterus development / vagina development / positive regulation of immunoglobulin production / response to retinoic acid / Retinoid metabolism and transport / retinol binding / Retinoid cycle disease events / The canonical retinoid cycle in rods (twilight vision) / uterus development / vagina development / positive regulation of immunoglobulin production / response to retinoic acid / Retinoid metabolism and transport /  visual perception / visual perception /  gluconeogenesis / lung development / positive regulation of insulin secretion / gluconeogenesis / lung development / positive regulation of insulin secretion /  glucose homeostasis / glucose homeostasis /  heart development / heart development /  extracellular space / extracellular exosome / extracellular region extracellular space / extracellular exosome / extracellular regionSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | |||||||||
 Authors Authors | Wang, Z. / Johnstone, S. / Walker, N.P. | |||||||||
 Citation Citation |  Journal: Bioorg.Med.Chem.Lett. / Year: 2014 Journal: Bioorg.Med.Chem.Lett. / Year: 2014Title: Structure-assisted discovery of the first non-retinoid ligands for Retinol-Binding Protein 4. Authors: Wang, Y. / Connors, R. / Fan, P. / Wang, X. / Wang, Z. / Liu, J. / Kayser, F. / Medina, J.C. / Johnstone, S. / Xu, H. / Thibault, S. / Walker, N. / Conn, M. / Zhang, Y. / Liu, Q. / Grillo, M. ...Authors: Wang, Y. / Connors, R. / Fan, P. / Wang, X. / Wang, Z. / Liu, J. / Kayser, F. / Medina, J.C. / Johnstone, S. / Xu, H. / Thibault, S. / Walker, N. / Conn, M. / Zhang, Y. / Liu, Q. / Grillo, M.P. / Motani, A. / Coward, P. / Wang, Z. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4o9s.cif.gz 4o9s.cif.gz | 90.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4o9s.ent.gz pdb4o9s.ent.gz | 72.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4o9s.json.gz 4o9s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/o9/4o9s https://data.pdbj.org/pub/pdb/validation_reports/o9/4o9s ftp://data.pdbj.org/pub/pdb/validation_reports/o9/4o9s ftp://data.pdbj.org/pub/pdb/validation_reports/o9/4o9s | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 24958.859 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: RBP4, PRO2222 / Production host: Homo sapiens (human) / Gene: RBP4, PRO2222 / Production host:   Escherichia coli (E. coli) / References: UniProt: P02753 Escherichia coli (E. coli) / References: UniProt: P02753 |
|---|
-Non-polymers , 5 types, 263 molecules 








| #2: Chemical | | #3: Chemical | ChemComp-EDO /  Ethylene glycol Ethylene glycol#4: Chemical |  Chloride Chloride#5: Chemical | ChemComp-PEG / |  Diethylene glycol Diethylene glycol#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.53 Å3/Da / Density % sol: 51.29 % |
|---|---|
Crystal grow | Temperature: 289 K / Method: vapor diffusion, sitting drop / pH: 8 Details: 1M ammonium phosphate dibasic, 0.1M imidazole, 0.2M sodium chloride, pH 8.0, VAPOR DIFFUSION, SITTING DROP, temperature 289K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.2 / Wavelength: 1 Å / Beamline: 5.0.2 / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jun 27, 2007 |
| Radiation | Monochromator: DOUBLE-CRYSTAL, SI (111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→63 Å / Num. all: 22054 / Num. obs: 22050 / % possible obs: 99.9 % / Observed criterion σ(F): 1 / Observed criterion σ(I): 2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 2.3→30 Å / Cor.coef. Fo:Fc: 0.922 / Cor.coef. Fo:Fc free: 0.894 / SU B: 7.852 / SU ML: 0.186 / Cross valid method: THROUGHOUT / ESU R: 0.345 / ESU R Free: 0.24 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS MOLECULAR REPLACEMENT / Resolution: 2.3→30 Å / Cor.coef. Fo:Fc: 0.922 / Cor.coef. Fo:Fc free: 0.894 / SU B: 7.852 / SU ML: 0.186 / Cross valid method: THROUGHOUT / ESU R: 0.345 / ESU R Free: 0.24 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 31.782 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→30 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj







