[English] 日本語
 Yorodumi
Yorodumi- PDB-4lxc: The antimicrobial peptidase lysostaphin from Staphylococcus simulans -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4lxc | ||||||
|---|---|---|---|---|---|---|---|
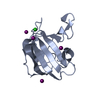
| Title | The antimicrobial peptidase lysostaphin from Staphylococcus simulans | ||||||
 Components Components | Lysostaphin | ||||||
 Keywords Keywords |  HYDROLASE / PEPTIDASE FAMILY M23 / PEPTIDOGLYCAN HYDROLASE / HYDROLASE / PEPTIDASE FAMILY M23 / PEPTIDOGLYCAN HYDROLASE /  METALLOPEPTIDASE / METALLOPEPTIDASE /  PEPTIDOGLYCAN PEPTIDOGLYCAN | ||||||
| Function / homology |  Function and homology information Function and homology information lysostaphin / cell wall organization / lysostaphin / cell wall organization /  metallopeptidase activity / metallopeptidase activity /  proteolysis / extracellular region / proteolysis / extracellular region /  metal ion binding metal ion bindingSimilarity search - Function | ||||||
| Biological species |   Staphylococcus simulans (bacteria) Staphylococcus simulans (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.5 Å MOLECULAR REPLACEMENT / Resolution: 3.5 Å | ||||||
 Authors Authors | Sabala, I. / Jagielska, E. / Bardelang, P.T. / Czapinska, H. / Dahms, S.O. / Sharpe, J.A. / James, R. / Than, M.E. / Thomas, N.R. / Bochtler, M. | ||||||
 Citation Citation |  Journal: Febs J. / Year: 2014 Journal: Febs J. / Year: 2014Title: Crystal structure of the antimicrobial peptidase lysostaphin from Staphylococcus simulans. Authors: Sabala, I. / Jagielska, E. / Bardelang, P.T. / Czapinska, H. / Dahms, S.O. / Sharpe, J.A. / James, R. / Than, M.E. / Thomas, N.R. / Bochtler, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4lxc.cif.gz 4lxc.cif.gz | 361 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4lxc.ent.gz pdb4lxc.ent.gz | 298.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4lxc.json.gz 4lxc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lx/4lxc https://data.pdbj.org/pub/pdb/validation_reports/lx/4lxc ftp://data.pdbj.org/pub/pdb/validation_reports/lx/4lxc ftp://data.pdbj.org/pub/pdb/validation_reports/lx/4lxc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4qp5C  4qpbC  1r77S  2b0pS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Refine code: 4
NCS ensembles :
|
- Components
Components
| #1: Protein |  / Glycyl-glycine endopeptidase / Glycyl-glycine endopeptidaseMass: 28177.551 Da / Num. of mol.: 4 / Fragment: unp residues 248-493 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Staphylococcus simulans (bacteria) / Strain: Staphylococcus simulans bv. staphylolyticus / Gene: lss, U66883.1 / Plasmid: pET21a / Production host: Staphylococcus simulans (bacteria) / Strain: Staphylococcus simulans bv. staphylolyticus / Gene: lss, U66883.1 / Plasmid: pET21a / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: P10547, Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: P10547,  lysostaphin lysostaphin#2: Chemical | ChemComp-ZN / #3: Chemical | ChemComp-SO4 /  Sulfate Sulfate |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 8.29 Å3/Da / Density % sol: 85.17 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 0.1 M MES and 1.6 M magnesium sulphate, 2 mM tetraglycine phosphinic acid. EDTA and ammonium sulphate as additives improved crystal quality, pH 6.5, VAPOR DIFFUSION, SITTING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.1 / Wavelength: 0.918409 / Wavelength: 0.918409 Å / Beamline: 14.1 / Wavelength: 0.918409 / Wavelength: 0.918409 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Feb 1, 2013 |
| Radiation | Monochromator: KMC-1 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.918409 Å / Relative weight: 1 : 0.918409 Å / Relative weight: 1 |
| Reflection | Resolution: 3.5→50 Å / Num. all: 48693 / Num. obs: 48693 / % possible obs: 100 % / Redundancy: 36.4 % / Biso Wilson estimate: 36.3 Å2 / Rmerge(I) obs: 0.295 / Rsym value: 0.295 / Net I/σ(I): 17.34 |
| Reflection shell | Resolution: 3.5→3.59 Å / Redundancy: 37.9 % / Rmerge(I) obs: 0.908 / Mean I/σ(I) obs: 5.34 / Num. unique all: 3539 / Rsym value: 0.908 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: pdb entries 1R77, 2B0P Resolution: 3.5→47.67 Å / Cor.coef. Fo:Fc: 0.823 / Cor.coef. Fo:Fc free: 0.796 / Cross valid method: THROUGHOUT / ESU R: 0.659 / ESU R Free: 0.414 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. NCS AND TLS REFINEMENT HAS BEEN USED. DEPENDING ON THE RESOLUTION CUTOFF WE OBSERVED A WILSON B VALUE IN THE RANGE OF 30 AND 80 AND KEPT ...Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. NCS AND TLS REFINEMENT HAS BEEN USED. DEPENDING ON THE RESOLUTION CUTOFF WE OBSERVED A WILSON B VALUE IN THE RANGE OF 30 AND 80 AND KEPT THE ATOMIC B AT A FIXED VALUE OF 36 OBSERVED FOR THE DEPOSITED DATA CUTOFF.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 40.566 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.5→47.67 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj


