+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4c6t | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the RPS4 and RRS1 TIR domain heterodimer | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  IMMUNE SYSTEM / PLANT TIR DOMAIN / IMMUNE SYSTEM / PLANT TIR DOMAIN /  SIGNAL TRANSDUCTION SIGNAL TRANSDUCTION | ||||||
| Function / homology |  Function and homology information Function and homology informationplant-type hypersensitive response / innate immune response-activating signaling pathway /  NAD+ nucleosidase activity / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NADP+ nucleosidase activity / NAD+ nucleotidase, cyclic ADP-ribose generating / NAD+ nucleosidase activity / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NADP+ nucleosidase activity / NAD+ nucleotidase, cyclic ADP-ribose generating /  endomembrane system / endomembrane system /  ADP binding / defense response / sequence-specific DNA binding ...plant-type hypersensitive response / innate immune response-activating signaling pathway / ADP binding / defense response / sequence-specific DNA binding ...plant-type hypersensitive response / innate immune response-activating signaling pathway /  NAD+ nucleosidase activity / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NADP+ nucleosidase activity / NAD+ nucleotidase, cyclic ADP-ribose generating / NAD+ nucleosidase activity / ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase / NADP+ nucleosidase activity / NAD+ nucleotidase, cyclic ADP-ribose generating /  endomembrane system / endomembrane system /  ADP binding / defense response / sequence-specific DNA binding / defense response to bacterium / DNA-binding transcription factor activity / ADP binding / defense response / sequence-specific DNA binding / defense response to bacterium / DNA-binding transcription factor activity /  signal transduction / signal transduction /  ATP binding / ATP binding /  membrane / identical protein binding / membrane / identical protein binding /  nucleus / nucleus /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   ARABIDOPSIS THALIANA (thale cress) ARABIDOPSIS THALIANA (thale cress) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.65 Å MOLECULAR REPLACEMENT / Resolution: 2.65 Å | ||||||
 Authors Authors | Williams, S.J. / Sohn, K.H. / Wan, L. / Bernoux, M. / Ma, Y. / Segonzac, C. / Ve, T. / Sarris, P. / Ericsson, D.J. / Saucet, S.B. ...Williams, S.J. / Sohn, K.H. / Wan, L. / Bernoux, M. / Ma, Y. / Segonzac, C. / Ve, T. / Sarris, P. / Ericsson, D.J. / Saucet, S.B. / Zhang, X. / Parker, J. / Dodds, P.N. / Jones, J.D.G. / Kobe, B. | ||||||
 Citation Citation |  Journal: Science / Year: 2014 Journal: Science / Year: 2014Title: Structural Basis for Assembly and Function of a Heterodimeric Plant Immune Receptor. Authors: Williams, S.J. / Sohn, K.H. / Wan, L. / Bernoux, M. / Sarris, P.F. / Segonzac, C. / Ve, T. / Ma, Y. / Saucet, S.B. / Ericsson, D.J. / Casey, L.W. / Lonhienne, T. / Winzor, D.J. / Zhang, X. / ...Authors: Williams, S.J. / Sohn, K.H. / Wan, L. / Bernoux, M. / Sarris, P.F. / Segonzac, C. / Ve, T. / Ma, Y. / Saucet, S.B. / Ericsson, D.J. / Casey, L.W. / Lonhienne, T. / Winzor, D.J. / Zhang, X. / Coerdt, A. / Parker, J.E. / Dodds, P.N. / Kobe, B. / Jones, J.D.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4c6t.cif.gz 4c6t.cif.gz | 263.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4c6t.ent.gz pdb4c6t.ent.gz | 219.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4c6t.json.gz 4c6t.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c6/4c6t https://data.pdbj.org/pub/pdb/validation_reports/c6/4c6t ftp://data.pdbj.org/pub/pdb/validation_reports/c6/4c6t ftp://data.pdbj.org/pub/pdb/validation_reports/c6/4c6t | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS oper:
|
- Components
Components
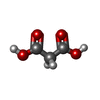
| #1: Protein | Mass: 17250.496 Da / Num. of mol.: 2 Fragment: TOLL/INTERLEUKIN-1 RECEPTOR DOMAIN, RESIDUES 6-153 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   ARABIDOPSIS THALIANA (thale cress) / Plasmid: PMCSG7 / Production host: ARABIDOPSIS THALIANA (thale cress) / Plasmid: PMCSG7 / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / Variant (production host): ROSETTA / References: UniProt: Q9FH83, UniProt: P0DKH5*PLUS ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / Variant (production host): ROSETTA / References: UniProt: Q9FH83, UniProt: P0DKH5*PLUS#2: Protein |  Mass: 19686.801 Da / Num. of mol.: 2 Fragment: TOLL/INTERLEUKIN-1 RECEPTOR DOMAIN, RESIDUES 11-178 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   ARABIDOPSIS THALIANA (thale cress) / Plasmid: PMCSG7 / Production host: ARABIDOPSIS THALIANA (thale cress) / Plasmid: PMCSG7 / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / Variant (production host): ROSETTA / References: UniProt: Q9XGM3 ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / Variant (production host): ROSETTA / References: UniProt: Q9XGM3#3: Chemical |  Malonic acid Malonic acid |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.57 Å3/Da / Density % sol: 65.58 % / Description: NONE |
|---|---|
Crystal grow | pH: 6 / Details: 1.8 M SODIUM MALONATE PH 6.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Australian Synchrotron Australian Synchrotron  / Beamline: MX2 / Wavelength: 0.9539 / Beamline: MX2 / Wavelength: 0.9539 |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Aug 29, 2012 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9539 Å / Relative weight: 1 : 0.9539 Å / Relative weight: 1 |
| Reflection | Resolution: 2.65→29.84 Å / Num. obs: 32619 / % possible obs: 99.9 % / Observed criterion σ(I): 2 / Redundancy: 23 % / Biso Wilson estimate: 73.73 Å2 / Rmerge(I) obs: 0.1 / Net I/σ(I): 27.5 |
| Reflection shell | Resolution: 2.65→2.78 Å / Redundancy: 24 % / Rmerge(I) obs: 1.5 / Mean I/σ(I) obs: 2 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: RPS4 TIR AND RRS1 TIR Resolution: 2.65→29.794 Å / SU ML: 0.36 / σ(F): 1.34 / Phase error: 21.13 / Stereochemistry target values: ML Details: CRYSTALS OF THE RRS1 AND RPS4 TIR DOMAIN HETERODIMER WERE OBTAINED BY LINKING RRS1(6-153) AND RPS4(10-178) WITH A FIVE-RESIDUE (GSGGS) LINKER . A REGION ENCOMPASSING 11 RESIDUES INCLUDING ...Details: CRYSTALS OF THE RRS1 AND RPS4 TIR DOMAIN HETERODIMER WERE OBTAINED BY LINKING RRS1(6-153) AND RPS4(10-178) WITH A FIVE-RESIDUE (GSGGS) LINKER . A REGION ENCOMPASSING 11 RESIDUES INCLUDING THE LINKER COULD NOT BE MODELED DUE TO THE LACK OF INTERPRETABLE ELECTRON DENSITY. WE FAVOR THE INTERPRETATION THAT THE LINKED CHAIN OCCURS BETWEEN MOLECULES A-D, B-C. HOWEVER, AS IT IS NOT POSSIBLE TO MODEL THE LINKER REGION WITH CERTAINTY WE CANNOT EXPLICITLY DETERMINE WHICH MOLECULES ARE LINKED IN THE CRYSTAL. FOR THIS REASON, WE HAVE LABELLED THE RRS1 AND RPS4 TIR DOMAIN MOLECULES AS SEPARATE CHAINS IN THE COORDINATE FILE DEPOSITED. THE FUNCTIONALLY IMPORTANT HETERODIMERISATION INTERFACE BETWEEN RRS1 AND RPS4 TIR DOMAINS OCCURS BETWEEN PROTEIN CHAINS A-B AND C-D.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.65→29.794 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj