[English] 日本語
 Yorodumi
Yorodumi- PDB-3gn9: Crystal structure of the major pseudopilin from the type 2 secret... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3gn9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the major pseudopilin from the type 2 secretion system of Vibrio vulnificus | ||||||
 Components Components | Type II secretory pathway, pseudopilin EpsG | ||||||
 Keywords Keywords |  PROTEIN TRANSPORT / GENERAL SECRETORY PATHWAY / MAJOR PILIN / PROTEIN TRANSPORT / GENERAL SECRETORY PATHWAY / MAJOR PILIN /  COMPLEX / COMPLEX /  Methylation Methylation | ||||||
| Function / homology |  Function and homology information Function and homology information protein secretion by the type II secretion system / protein secretion by the type II secretion system /  type II protein secretion system complex / membrane => GO:0016020 / type II protein secretion system complex / membrane => GO:0016020 /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Vibrio vulnificus (bacteria) Vibrio vulnificus (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.86 Å molecular replacement / Resolution: 1.86 Å | ||||||
 Authors Authors | Korotkov, K.V. / Gray, M.D. / Kreger, A. / Turley, S. / Sandkvist, M. / Hol, W.G.J. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2009 Journal: J.Biol.Chem. / Year: 2009Title: Calcium is essential for the major pseudopilin in the type 2 secretion system. Authors: Korotkov, K.V. / Gray, M.D. / Kreger, A. / Turley, S. / Sandkvist, M. / Hol, W.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3gn9.cif.gz 3gn9.cif.gz | 86.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3gn9.ent.gz pdb3gn9.ent.gz | 64.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3gn9.json.gz 3gn9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gn/3gn9 https://data.pdbj.org/pub/pdb/validation_reports/gn/3gn9 ftp://data.pdbj.org/pub/pdb/validation_reports/gn/3gn9 ftp://data.pdbj.org/pub/pdb/validation_reports/gn/3gn9 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3fu1C  3g20C  1fu1S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 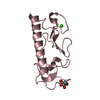
| ||||||||
| Unit cell |
| ||||||||
| Details | THIS CRYSTAL FORM CONTAINS THREE MONOMERS, THAT ARE NOT RELATED TO THE BIOLOGICAL FORM. THE BIOLOGICAL UNIT IS A FIBER. IT IS UNKNOWN AT THE MOMENT HOW INDIVIDUAL SUBUNITS ARE ASSEMBLED IN VIVO. |
- Components
Components
| #1: Protein | Mass: 12585.797 Da / Num. of mol.: 3 / Fragment: UNP residues 26-137 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Vibrio vulnificus (bacteria) / Strain: MO6-24 / Gene: EPSG, VV1_0874 / Plasmid: pCDF-NT / Production host: Vibrio vulnificus (bacteria) / Strain: MO6-24 / Gene: EPSG, VV1_0874 / Plasmid: pCDF-NT / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q8DDT3, UniProt: A0A3Q0L2Q1*PLUS Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q8DDT3, UniProt: A0A3Q0L2Q1*PLUS#2: Chemical | #3: Chemical |  Malic acid Malic acid#4: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.69 Å3/Da / Density % sol: 66.66 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, sitting drop / pH: 5 Details: 1.95M DL-Malic acid pH 5.0, VAPOR DIFFUSION, SITTING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL9-2 / Wavelength: 1.5418 Å / Beamline: BL9-2 / Wavelength: 1.5418 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: MARMOSAIC 325 mm CCD / Detector: CCD / Date: Dec 3, 2008 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Double crystal Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.86→38.9 Å / Num. all: 90808 / Num. obs: 78730 / % possible obs: 86.7 % / Observed criterion σ(I): -3 / Redundancy: 2.1 % / Biso Wilson estimate: 38.722 Å2 / Rmerge(I) obs: 0.029 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
-Phasing
Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR | Rfactor: 38.73 / Model details: Phaser MODE: MR_AUTO
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 1FU1 Resolution: 1.86→38.9 Å / Cor.coef. Fo:Fc: 0.969 / Cor.coef. Fo:Fc free: 0.96 / WRfactor Rfree: 0.203 / WRfactor Rwork: 0.178 / Occupancy max: 1 / Occupancy min: 0.5 / FOM work R set: 0.821 / SU B: 7.356 / SU ML: 0.096 / SU R Cruickshank DPI: 0.105 / SU Rfree: 0.102 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.105 / ESU R Free: 0.102 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: 1. The Friedel pairs were used in phasing. 2. HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. 3. U VALUES: RESIDUAL ONLY.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 71.27 Å2 / Biso mean: 34.969 Å2 / Biso min: 13.33 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.86→38.9 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.86→1.908 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj







