| Entry | Database: PDB / ID: 2vh1
|
|---|
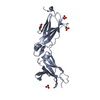
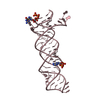
| Title | Crystal structure of bacterial cell division protein FtsQ from E.coli |
|---|
 Components Components | CELL DIVISION PROTEIN FTSQ |
|---|
 Keywords Keywords |  CELL CYCLE / FTSQ / CELL CYCLE / FTSQ /  POTRA / POTRA /  MEMBRANE / MEMBRANE /  SEPTATION / SEPTATION /  CELL DIVISION / CELL DIVISION /  TRANSMEMBRANE / INNER MEMBRANE TRANSMEMBRANE / INNER MEMBRANE |
|---|
| Function / homology |  Function and homology information Function and homology information
FtsQBL complex /  divisome complex / FtsZ-dependent cytokinesis / division septum assembly / cell division site / divisome complex / FtsZ-dependent cytokinesis / division septum assembly / cell division site /  cell division / identical protein binding / cell division / identical protein binding /  plasma membraneSimilarity search - Function plasma membraneSimilarity search - Function  Cell division protein FtsQ/DivIB / Cell division protein FtsQ/DivIB /  Cell division protein FtsQ, C-terminal / Cell division protein FtsQ, C-terminal /  Cell division protein FtsQ / Cell division protein FtsQ /  Cell division protein FtsQ/DivIB, C-terminal / POTRA domain, FtsQ-type / Cell division protein FtsQ/DivIB, C-terminal / POTRA domain, FtsQ-type /  Cell division protein FtsQ/DivIB, C-terminal / POTRA domain, FtsQ-type / Cell division protein FtsQ/DivIB, C-terminal / POTRA domain, FtsQ-type /  membrane protein fhac / membrane protein fhac /  POTRA domain / POTRA domain profile. ... POTRA domain / POTRA domain profile. ... Cell division protein FtsQ/DivIB / Cell division protein FtsQ/DivIB /  Cell division protein FtsQ, C-terminal / Cell division protein FtsQ, C-terminal /  Cell division protein FtsQ / Cell division protein FtsQ /  Cell division protein FtsQ/DivIB, C-terminal / POTRA domain, FtsQ-type / Cell division protein FtsQ/DivIB, C-terminal / POTRA domain, FtsQ-type /  Cell division protein FtsQ/DivIB, C-terminal / POTRA domain, FtsQ-type / Cell division protein FtsQ/DivIB, C-terminal / POTRA domain, FtsQ-type /  membrane protein fhac / membrane protein fhac /  POTRA domain / POTRA domain profile. / Ubiquitin-like (UB roll) / Roll / POTRA domain / POTRA domain profile. / Ubiquitin-like (UB roll) / Roll /  Rossmann fold / 3-Layer(aba) Sandwich / Alpha BetaSimilarity search - Domain/homology Rossmann fold / 3-Layer(aba) Sandwich / Alpha BetaSimilarity search - Domain/homology |
|---|
| Biological species |    ESCHERICHIA COLI (E. coli) ESCHERICHIA COLI (E. coli) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 2.7 Å MAD / Resolution: 2.7 Å |
|---|
 Authors Authors | van den Ent, F. / Vinkenvleugel, T. / Ind, A. / West, P. / Veprintsev, D. / Naninga, N. / den Blaauwen, T. / Lowe, J. |
|---|
 Citation Citation |  Journal: Mol.Microbiol. / Year: 2008 Journal: Mol.Microbiol. / Year: 2008
Title: Structural and Mutational Analysis of Cell Division Protein Ftsq
Authors: van den Ent, F. / Vinkenvleugel, T. / Ind, A. / West, P. / Veprintsev, D. / Naninga, N. / den Blaauwen, T. / Lowe, J. |
|---|
| History | | Deposition | Nov 16, 2007 | Deposition site: PDBE / Processing site: PDBE |
|---|
| Revision 1.0 | Mar 11, 2008 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Jul 13, 2011 | Group: Advisory / Version format compliance |
|---|
| Revision 1.2 | Jan 24, 2018 | Group: Database references / Source and taxonomy / Category: citation_author / entity_src_gen
Item: _citation_author.name / _entity_src_gen.pdbx_host_org_ncbi_taxonomy_id ..._citation_author.name / _entity_src_gen.pdbx_host_org_ncbi_taxonomy_id / _entity_src_gen.pdbx_host_org_scientific_name / _entity_src_gen.pdbx_host_org_strain / _entity_src_gen.pdbx_host_org_variant |
|---|
| Revision 1.3 | May 8, 2024 | Group: Data collection / Database references ...Data collection / Database references / Other / Refinement description
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_database_status / struct_ncs_dom_lim
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_sf / _struct_ncs_dom_lim.beg_auth_comp_id / _struct_ncs_dom_lim.beg_label_asym_id / _struct_ncs_dom_lim.beg_label_comp_id / _struct_ncs_dom_lim.beg_label_seq_id / _struct_ncs_dom_lim.end_auth_comp_id / _struct_ncs_dom_lim.end_label_asym_id / _struct_ncs_dom_lim.end_label_comp_id / _struct_ncs_dom_lim.end_label_seq_id |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components
 Keywords
Keywords CELL CYCLE / FTSQ /
CELL CYCLE / FTSQ /  POTRA /
POTRA /  MEMBRANE /
MEMBRANE /  SEPTATION /
SEPTATION /  CELL DIVISION /
CELL DIVISION /  TRANSMEMBRANE / INNER MEMBRANE
TRANSMEMBRANE / INNER MEMBRANE Function and homology information
Function and homology information divisome complex / FtsZ-dependent cytokinesis / division septum assembly / cell division site /
divisome complex / FtsZ-dependent cytokinesis / division septum assembly / cell division site /  cell division / identical protein binding /
cell division / identical protein binding /  plasma membrane
plasma membrane
 ESCHERICHIA COLI (E. coli)
ESCHERICHIA COLI (E. coli) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MAD / Resolution: 2.7 Å
MAD / Resolution: 2.7 Å  Authors
Authors Citation
Citation Journal: Mol.Microbiol. / Year: 2008
Journal: Mol.Microbiol. / Year: 2008 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2vh1.cif.gz
2vh1.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2vh1.ent.gz
pdb2vh1.ent.gz PDB format
PDB format 2vh1.json.gz
2vh1.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/vh/2vh1
https://data.pdbj.org/pub/pdb/validation_reports/vh/2vh1 ftp://data.pdbj.org/pub/pdb/validation_reports/vh/2vh1
ftp://data.pdbj.org/pub/pdb/validation_reports/vh/2vh1 Links
Links Assembly
Assembly


 :
:  Components
Components / FTSQ
/ FTSQ
 ESCHERICHIA COLI (E. coli) / Plasmid: PHIS17 / Production host:
ESCHERICHIA COLI (E. coli) / Plasmid: PHIS17 / Production host: 
 ESCHERICHIA COLI BL21(DE3) (bacteria) / Variant (production host): C41 / References: UniProt: P06136
ESCHERICHIA COLI BL21(DE3) (bacteria) / Variant (production host): C41 / References: UniProt: P06136 X-RAY DIFFRACTION
X-RAY DIFFRACTION Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID23-1 / Wavelength: 0.9392
/ Beamline: ID23-1 / Wavelength: 0.9392  : 0.9392 Å / Relative weight: 1
: 0.9392 Å / Relative weight: 1  Processing
Processing :
:  MAD
MAD Movie
Movie Controller
Controller











 PDBj
PDBj