[English] 日本語
 Yorodumi
Yorodumi- PDB-2uuh: Crystal structure of Human Leukotriene C4 Synthase in complex wit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2uuh | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Human Leukotriene C4 Synthase in complex with substrate glutathione | ||||||
 Components Components | LEUKOTRIENE C4 SYNTHASE | ||||||
 Keywords Keywords |  LYASE / LYASE /  MEMBRANE PROTEIN / LEUKOTRIENE SIGNALLING / MEMBRANE PROTEIN / LEUKOTRIENE SIGNALLING /  MAPEG / MAPEG /  HUMAN / HUMAN /  LTC4S / LTC4S /  ENZYME / TRIMER / ENZYME / TRIMER /  MEMBRANE / LEUKOTRIENE BIOSYNTHESIS / MEMBRANE / LEUKOTRIENE BIOSYNTHESIS /  EICOSANOID / EICOSANOID /  GLUTATHIONE / GLUTATHIONE /  TRANSMEMBRANE TRANSMEMBRANE | ||||||
| Function / homology |  Function and homology information Function and homology informationBiosynthesis of protectin and resolvin conjugates in tissue regeneration (PCTR and RCTR) / Biosynthesis of maresin conjugates in tissue regeneration (MCTR) /  leukotriene-C4 synthase / leukotriene-C4 synthase /  leukotriene-C4 synthase activity / Synthesis of Lipoxins (LX) / Synthesis of 5-eicosatetraenoic acids / leukotriene metabolic process / leukotriene-C4 synthase activity / Synthesis of Lipoxins (LX) / Synthesis of 5-eicosatetraenoic acids / leukotriene metabolic process /  Transferases; Transferring alkyl or aryl groups, other than methyl groups / Synthesis of Leukotrienes (LT) and Eoxins (EX) / leukotriene biosynthetic process ...Biosynthesis of protectin and resolvin conjugates in tissue regeneration (PCTR and RCTR) / Biosynthesis of maresin conjugates in tissue regeneration (MCTR) / Transferases; Transferring alkyl or aryl groups, other than methyl groups / Synthesis of Leukotrienes (LT) and Eoxins (EX) / leukotriene biosynthetic process ...Biosynthesis of protectin and resolvin conjugates in tissue regeneration (PCTR and RCTR) / Biosynthesis of maresin conjugates in tissue regeneration (MCTR) /  leukotriene-C4 synthase / leukotriene-C4 synthase /  leukotriene-C4 synthase activity / Synthesis of Lipoxins (LX) / Synthesis of 5-eicosatetraenoic acids / leukotriene metabolic process / leukotriene-C4 synthase activity / Synthesis of Lipoxins (LX) / Synthesis of 5-eicosatetraenoic acids / leukotriene metabolic process /  Transferases; Transferring alkyl or aryl groups, other than methyl groups / Synthesis of Leukotrienes (LT) and Eoxins (EX) / leukotriene biosynthetic process / Transferases; Transferring alkyl or aryl groups, other than methyl groups / Synthesis of Leukotrienes (LT) and Eoxins (EX) / leukotriene biosynthetic process /  glutathione peroxidase activity / nuclear outer membrane / long-chain fatty acid biosynthetic process / glutathione peroxidase activity / nuclear outer membrane / long-chain fatty acid biosynthetic process /  glutathione transferase activity / glutathione transferase activity /  enzyme activator activity / enzyme activator activity /  nuclear envelope / nuclear envelope /  nuclear membrane / intracellular membrane-bounded organelle / nuclear membrane / intracellular membrane-bounded organelle /  lipid binding / endoplasmic reticulum membrane / lipid binding / endoplasmic reticulum membrane /  endoplasmic reticulum / endoplasmic reticulum /  membrane / identical protein binding membrane / identical protein bindingSimilarity search - Function | ||||||
| Biological species |   HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.15 Å MOLECULAR REPLACEMENT / Resolution: 2.15 Å | ||||||
 Authors Authors | Martinez Molina, D. / Wetterholm, A. / Kohl, A. / McCarthy, A.A. / Niegowski, D. / Ohlson, E. / Hammarberg, T. / Eshaghi, S. / Haeggstrom, J.Z. / Nordlund, P. | ||||||
 Citation Citation |  Journal: Nature / Year: 2007 Journal: Nature / Year: 2007Title: Structural Basis for Synthesis of Inflammatory Mediators by Human Leukotriene C4 Synthase. Authors: Martinez Molina, D. / Wetterholm, A. / Kohl, A. / Mccarthy, A.A. / Niegowski, D. / Ohlson, E. / Hammarberg, T. / Eshaghi, S. / Haeggstrom, J.Z. / Nordlund, P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2uuh.cif.gz 2uuh.cif.gz | 53.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2uuh.ent.gz pdb2uuh.ent.gz | 37.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2uuh.json.gz 2uuh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uu/2uuh https://data.pdbj.org/pub/pdb/validation_reports/uu/2uuh ftp://data.pdbj.org/pub/pdb/validation_reports/uu/2uuh ftp://data.pdbj.org/pub/pdb/validation_reports/uu/2uuh | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 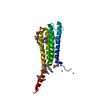
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Sugars , 2 types, 2 molecules A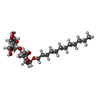

| #1: Protein |  / LEUKOTRIENE-C(4) SYNTHASE / LTC4 SYNTHASE / LEUKOTRIENE-C(4) SYNTHASE / LTC4 SYNTHASEMass: 17411.545 Da / Num. of mol.: 1 / Fragment: RESIDUES 2-150 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   HOMO SAPIENS (human) / Plasmid: PPICZ / Production host: HOMO SAPIENS (human) / Plasmid: PPICZ / Production host:   PICHIA PASTORIS (fungus) / Strain (production host): KM71H / References: UniProt: Q16873, PICHIA PASTORIS (fungus) / Strain (production host): KM71H / References: UniProt: Q16873,  leukotriene-C4 synthase leukotriene-C4 synthase |
|---|---|
| #3: Sugar | ChemComp-LMU / |
-Non-polymers , 6 types, 132 molecules 

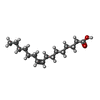








| #2: Chemical |  Nickel Nickel#4: Chemical | ChemComp-GSH / |  Glutathione Glutathione#5: Chemical | ChemComp-PAM / |  Palmitoleic acid Palmitoleic acid#6: Chemical | ChemComp-PLM /  Palmitic acid Palmitic acid#7: Chemical | ChemComp-SO4 / |  Sulfate Sulfate#8: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 5.07 Å3/Da / Density % sol: 80 % / Description: NONE |
|---|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-2 / Wavelength: 0.8733 / Beamline: ID23-2 / Wavelength: 0.8733 |
| Detector | Type: MARRESEARCH / Detector: CCD |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.8733 Å / Relative weight: 1 : 0.8733 Å / Relative weight: 1 |
| Reflection | Resolution: 1.51→51.23 Å / Num. obs: 47317 / % possible obs: 99.8 % / Redundancy: 5 % / Rmerge(I) obs: 0.14 / Net I/σ(I): 8.8 |
| Reflection shell | Highest resolution: 2.15 Å / Redundancy: 5 % / % possible all: 100 |
- Processing
Processing
| Software | Name: REFMAC / Version: 5.2.0019 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 2.15→49.03 Å / Cor.coef. Fo:Fc: 0.942 / Cor.coef. Fo:Fc free: 0.921 / SU B: 7.431 / SU ML: 0.099 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / ESU R: 0.124 / ESU R Free: 0.127 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS MOLECULAR REPLACEMENT / Resolution: 2.15→49.03 Å / Cor.coef. Fo:Fc: 0.942 / Cor.coef. Fo:Fc free: 0.921 / SU B: 7.431 / SU ML: 0.099 / TLS residual ADP flag: LIKELY RESIDUAL / Cross valid method: THROUGHOUT / ESU R: 0.124 / ESU R Free: 0.127 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 21.52 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.15→49.03 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj











