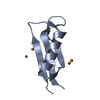
Entry Database : PDB / ID : 2fmaTitle Structure of the Alzheimer's Amyloid Precursor Protein (APP) Copper Binding Domain in 'small unit cell' form, atomic resolution Amyloid beta A4 protein precursor Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 0.85 Å Authors Kong, G.K.-W. Journal : Acta Crystallogr.,Sect.F / Year : 2007Title : Structure of Alzheimer's disease amyloid precursor protein copper-binding domain at atomic resolution.Authors : Kong, G.K. / Adams, J.J. / Cappai, R. / Parker, M.W. History Deposition Jan 8, 2006 Deposition site / Processing site Revision 1.0 Jan 16, 2007 Provider / Type Revision 1.1 May 1, 2008 Group Revision 1.2 Jul 13, 2011 Group / Version format complianceRevision 1.3 Oct 25, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / struct_ref_seq_dif / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information regulation of Wnt signaling pathway / mating behavior /
regulation of Wnt signaling pathway / mating behavior /  ciliary rootlet / Lysosome Vesicle Biogenesis /
ciliary rootlet / Lysosome Vesicle Biogenesis /  PTB domain binding / Golgi-associated vesicle / positive regulation of amyloid fibril formation / neuron remodeling / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / : / Deregulated CDK5 triggers multiple neurodegenerative pathways in Alzheimer's disease models / presynaptic active zone / nuclear envelope lumen / modulation of excitatory postsynaptic potential / suckling behavior / COPII-coated ER to Golgi transport vesicle / dendrite development /
PTB domain binding / Golgi-associated vesicle / positive regulation of amyloid fibril formation / neuron remodeling / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / : / Deregulated CDK5 triggers multiple neurodegenerative pathways in Alzheimer's disease models / presynaptic active zone / nuclear envelope lumen / modulation of excitatory postsynaptic potential / suckling behavior / COPII-coated ER to Golgi transport vesicle / dendrite development /  smooth endoplasmic reticulum /
smooth endoplasmic reticulum /  regulation of NMDA receptor activity / TRAF6 mediated NF-kB activation / Advanced glycosylation endproduct receptor signaling / neuromuscular process controlling balance / regulation of presynapse assembly / The NLRP3 inflammasome / intracellular copper ion homeostasis / transition metal ion binding / regulation of multicellular organism growth / negative regulation of long-term synaptic potentiation / negative regulation of neuron differentiation / ECM proteoglycans / spindle midzone / positive regulation of T cell migration /
regulation of NMDA receptor activity / TRAF6 mediated NF-kB activation / Advanced glycosylation endproduct receptor signaling / neuromuscular process controlling balance / regulation of presynapse assembly / The NLRP3 inflammasome / intracellular copper ion homeostasis / transition metal ion binding / regulation of multicellular organism growth / negative regulation of long-term synaptic potentiation / negative regulation of neuron differentiation / ECM proteoglycans / spindle midzone / positive regulation of T cell migration /  Purinergic signaling in leishmaniasis infection / positive regulation of calcium-mediated signaling / forebrain development / regulation of peptidyl-tyrosine phosphorylation / positive regulation of chemokine production /
Purinergic signaling in leishmaniasis infection / positive regulation of calcium-mediated signaling / forebrain development / regulation of peptidyl-tyrosine phosphorylation / positive regulation of chemokine production /  clathrin-coated pit /
clathrin-coated pit /  Notch signaling pathway / positive regulation of G2/M transition of mitotic cell cycle / ionotropic glutamate receptor signaling pathway / positive regulation of protein metabolic process / neuron projection maintenance / cholesterol metabolic process / extracellular matrix organization / positive regulation of glycolytic process / positive regulation of mitotic cell cycle / response to interleukin-1 /
Notch signaling pathway / positive regulation of G2/M transition of mitotic cell cycle / ionotropic glutamate receptor signaling pathway / positive regulation of protein metabolic process / neuron projection maintenance / cholesterol metabolic process / extracellular matrix organization / positive regulation of glycolytic process / positive regulation of mitotic cell cycle / response to interleukin-1 /  axonogenesis / adult locomotory behavior / trans-Golgi network membrane / dendritic shaft / locomotory behavior / platelet alpha granule lumen / positive regulation of peptidyl-threonine phosphorylation /
axonogenesis / adult locomotory behavior / trans-Golgi network membrane / dendritic shaft / locomotory behavior / platelet alpha granule lumen / positive regulation of peptidyl-threonine phosphorylation /  learning /
learning /  central nervous system development / positive regulation of interleukin-1 beta production / positive regulation of long-term synaptic potentiation / astrocyte activation / endosome lumen / synapse organization /
central nervous system development / positive regulation of interleukin-1 beta production / positive regulation of long-term synaptic potentiation / astrocyte activation / endosome lumen / synapse organization /  Post-translational protein phosphorylation / regulation of long-term neuronal synaptic plasticity / positive regulation of JNK cascade / microglial cell activation / TAK1-dependent IKK and NF-kappa-B activation /
Post-translational protein phosphorylation / regulation of long-term neuronal synaptic plasticity / positive regulation of JNK cascade / microglial cell activation / TAK1-dependent IKK and NF-kappa-B activation /  visual learning /
visual learning /  neuromuscular junction / serine-type endopeptidase inhibitor activity / recycling endosome /
neuromuscular junction / serine-type endopeptidase inhibitor activity / recycling endosome /  cognition / positive regulation of inflammatory response / Golgi lumen / neuron cellular homeostasis /
cognition / positive regulation of inflammatory response / Golgi lumen / neuron cellular homeostasis /  endocytosis / positive regulation of interleukin-6 production / positive regulation of non-canonical NF-kappaB signal transduction / cellular response to amyloid-beta / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / neuron projection development / positive regulation of DNA-binding transcription factor activity / G2/M transition of mitotic cell cycle / cell-cell junction /
endocytosis / positive regulation of interleukin-6 production / positive regulation of non-canonical NF-kappaB signal transduction / cellular response to amyloid-beta / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / neuron projection development / positive regulation of DNA-binding transcription factor activity / G2/M transition of mitotic cell cycle / cell-cell junction /  synaptic vesicle / positive regulation of tumor necrosis factor production /
synaptic vesicle / positive regulation of tumor necrosis factor production /  regulation of translation
regulation of translation
 Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON / Rigid body refinement / Resolution: 0.85 Å
SYNCHROTRON / Rigid body refinement / Resolution: 0.85 Å  Authors
Authors Citation
Citation Journal: Acta Crystallogr.,Sect.F / Year: 2007
Journal: Acta Crystallogr.,Sect.F / Year: 2007 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2fma.cif.gz
2fma.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2fma.ent.gz
pdb2fma.ent.gz PDB format
PDB format 2fma.json.gz
2fma.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/fm/2fma
https://data.pdbj.org/pub/pdb/validation_reports/fm/2fma ftp://data.pdbj.org/pub/pdb/validation_reports/fm/2fma
ftp://data.pdbj.org/pub/pdb/validation_reports/fm/2fma
 Links
Links Assembly
Assembly
 Components
Components
 Homo sapiens (human) / Plasmid: pPIC-9 / Production host:
Homo sapiens (human) / Plasmid: pPIC-9 / Production host: 
 Pichia pastoris (fungus) / Strain (production host): GS115 / References: UniProt: P05067
Pichia pastoris (fungus) / Strain (production host): GS115 / References: UniProt: P05067 Glycerol
Glycerol Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 14-BM-C / Wavelength: 0.9 Å
/ Beamline: 14-BM-C / Wavelength: 0.9 Å : 0.9 Å / Relative weight: 1
: 0.9 Å / Relative weight: 1  Processing
Processing : Rigid body refinement
: Rigid body refinement Movie
Movie Controller
Controller











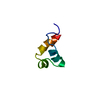

 PDBj
PDBj

















