[English] 日本語
 Yorodumi
Yorodumi- PDB-2f0y: Crystal Structure Of Human Protein Farnesyltransferase Complexed ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2f0y | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure Of Human Protein Farnesyltransferase Complexed With Farnesyl Diphosphate and hydantoin derivative | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  farnesyltransferase farnesyltransferase | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of deacetylase activity / skeletal muscle acetylcholine-gated channel clustering / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex /  protein geranylgeranyltransferase type I / protein farnesylation / positive regulation of tubulin deacetylation / protein geranylgeranyltransferase type I / protein farnesylation / positive regulation of tubulin deacetylation /  protein farnesyltransferase / protein farnesyltransferase /  protein farnesyltransferase activity ...positive regulation of deacetylase activity / skeletal muscle acetylcholine-gated channel clustering / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein farnesyltransferase activity ...positive regulation of deacetylase activity / skeletal muscle acetylcholine-gated channel clustering / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex /  protein geranylgeranyltransferase type I / protein farnesylation / positive regulation of tubulin deacetylation / protein geranylgeranyltransferase type I / protein farnesylation / positive regulation of tubulin deacetylation /  protein farnesyltransferase / protein farnesyltransferase /  protein farnesyltransferase activity / protein farnesyltransferase activity /  protein farnesyltransferase complex / protein farnesyltransferase complex /  Rab geranylgeranyltransferase activity / protein geranylgeranylation / positive regulation of skeletal muscle acetylcholine-gated channel clustering / microtubule associated complex / Apoptotic cleavage of cellular proteins / positive regulation of Rac protein signal transduction / alpha-tubulin binding / transforming growth factor beta receptor signaling pathway / lipid metabolic process / Rab geranylgeranyltransferase activity / protein geranylgeranylation / positive regulation of skeletal muscle acetylcholine-gated channel clustering / microtubule associated complex / Apoptotic cleavage of cellular proteins / positive regulation of Rac protein signal transduction / alpha-tubulin binding / transforming growth factor beta receptor signaling pathway / lipid metabolic process /  receptor tyrosine kinase binding / RAS processing / Inactivation, recovery and regulation of the phototransduction cascade / receptor tyrosine kinase binding / RAS processing / Inactivation, recovery and regulation of the phototransduction cascade /  microtubule binding / Potential therapeutics for SARS / molecular adaptor activity / zinc ion binding / microtubule binding / Potential therapeutics for SARS / molecular adaptor activity / zinc ion binding /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.7 Å MOLECULAR REPLACEMENT / Resolution: 2.7 Å | ||||||
 Authors Authors | Kim, K.H. / Lee, J. / Kim, J. | ||||||
 Citation Citation |  Journal: To be published Journal: To be publishedTitle: hydantoin derivatives as Non-pepridic inhibitors of Ras Farnesyl transferase Authors: Lee, J. / Kim, J. / Koh, J.S. / Chung, H.H. / Ro, S. / Kim, K.H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2f0y.cif.gz 2f0y.cif.gz | 156.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2f0y.ent.gz pdb2f0y.ent.gz | 122 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2f0y.json.gz 2f0y.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f0/2f0y https://data.pdbj.org/pub/pdb/validation_reports/f0/2f0y ftp://data.pdbj.org/pub/pdb/validation_reports/f0/2f0y ftp://data.pdbj.org/pub/pdb/validation_reports/f0/2f0y | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | The biological assembly is the dimer of alpha and beta subunits. |
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 44459.441 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: P49354,  protein farnesyltransferase, protein farnesyltransferase,  protein geranylgeranyltransferase type I protein geranylgeranyltransferase type I |
|---|---|
| #2: Protein | Mass: 48822.406 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:   Escherichia coli (E. coli) / References: UniProt: P49356, Escherichia coli (E. coli) / References: UniProt: P49356,  protein farnesyltransferase protein farnesyltransferase |
-Non-polymers , 4 types, 412 molecules 
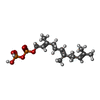
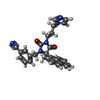




| #3: Chemical | ChemComp-ZN / |
|---|---|
| #4: Chemical | ChemComp-FPP /  Farnesyl pyrophosphate Farnesyl pyrophosphate |
| #5: Chemical | ChemComp-3MN / |
| #6: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.26 Å3/Da / Density % sol: 62.28 % |
|---|---|
Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 5.3 Details: 0.1M Na Aetate, pH 5.1, 0.2M NaCl, 10% isopropyl alcohol , pH 5.3, VAPOR DIFFUSION, HANGING DROP, temperature 295K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: PAL/PLS SYNCHROTRON / Site: PAL/PLS  / Beamline: 6B / Wavelength: 1 Å / Beamline: 6B / Wavelength: 1 Å |
| Detector | Type: MACSCIENCE / Detector: IMAGE PLATE / Date: Sep 20, 2001 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.7→30 Å / Num. all: 33313 / Num. obs: 29887 / % possible obs: 98.7 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Rsym value: 0.055 |
| Reflection shell | Resolution: 2.7→2.75 Å / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 2.7→30 Å / σ(F): 0 / Stereochemistry target values: Engh & Huber MOLECULAR REPLACEMENT / Resolution: 2.7→30 Å / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.7→30 Å
| ||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj








