[English] 日本語
 Yorodumi
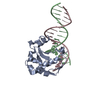
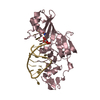
Yorodumi- PDB-1wvr: Crystal Structure of a CRISP family Ca-channel blocker derived fr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1wvr | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of a CRISP family Ca-channel blocker derived from snake venom | ||||||
 Components Components | Triflin | ||||||
 Keywords Keywords |  TOXIN / TOXIN /  cysteine-rich secretory protein cysteine-rich secretory protein | ||||||
| Function / homology |  Function and homology information Function and homology informationcalcium channel regulator activity /  toxin activity / extracellular region toxin activity / extracellular regionSimilarity search - Function | ||||||
| Biological species |   Trimeresurus flavoviridis (habu) Trimeresurus flavoviridis (habu) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Crystal 1 SINGLE WAVELENGTH PROTOCOL, Crysatl 2 MAD PROTOCOL / Resolution: 2.4 Å SYNCHROTRON / Crystal 1 SINGLE WAVELENGTH PROTOCOL, Crysatl 2 MAD PROTOCOL / Resolution: 2.4 Å | ||||||
 Authors Authors | Shikamoto, Y. / Suto, K. / Yamazaki, Y. / Morita, T. / Mizuno, H. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2005 Journal: J.Mol.Biol. / Year: 2005Title: Crystal structure of a CRISP family Ca2+ -channel blocker derived from snake venom. Authors: Shikamoto, Y. / Suto, K. / Yamazaki, Y. / Morita, T. / Mizuno, H. #1: Journal: Eur.J.Biochem. / Year: 2002 Title: Cloning and characterization of novel snake venom proteins that block smooth muscle contraction Authors: Yamazaki, Y. / Koike, H. / Sugiyama, Y. / Motoyoshi, K. / Wada, T. / Hishinuma, S. / Mita, M. / Morita, T. #2: Journal: Arch.Biochem.Biophys. / Year: 2003 Title: Wide distribution of cysteine-rich secretory proteins in snake venoms: isolation and cloning of novel snake venom cysteine-rich secretory proteins Authors: Yamazaki, Y. / Hyodo, F. / Morita, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1wvr.cif.gz 1wvr.cif.gz | 56.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1wvr.ent.gz pdb1wvr.ent.gz | 45.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1wvr.json.gz 1wvr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wv/1wvr https://data.pdbj.org/pub/pdb/validation_reports/wv/1wvr ftp://data.pdbj.org/pub/pdb/validation_reports/wv/1wvr ftp://data.pdbj.org/pub/pdb/validation_reports/wv/1wvr | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 24826.912 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Trimeresurus flavoviridis (habu) / Strain: Tokunoshima / References: UniProt: Q8JI39 Trimeresurus flavoviridis (habu) / Strain: Tokunoshima / References: UniProt: Q8JI39 | ||
|---|---|---|---|
| #2: Chemical | ChemComp-CD / #3: Water | ChemComp-HOH / |  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 2 X-RAY DIFFRACTION / Number of used crystals: 2 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.1 Å3/Da / Density % sol: 60 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 8 Details: Tris-HCl, PEG 400, sodium acetate, sodium cadmium, pH 8.0, VAPOR DIFFUSION, HANGING DROP, temperature 293K |
-Data collection
| Diffraction |
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| ||||||||||||||||||
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Dec 2, 2002 / Details: mirrors | ||||||||||||||||||
| Radiation |
| ||||||||||||||||||
| Radiation wavelength |
| ||||||||||||||||||
| Reflection | Resolution: 2.3→38.11 Å / Num. all: 14053 / Num. obs: 13969 / % possible obs: 99.4 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Biso Wilson estimate: 46.1 Å2 / Rmerge(I) obs: 0.063 / Rsym value: 0.063 / Net I/σ(I): 8.25 | ||||||||||||||||||
| Reflection shell | Resolution: 2.3→2.42 Å / Rmerge(I) obs: 0.518 / Mean I/σ(I) obs: 1.6 / Num. unique all: 1965 / Rsym value: 0.48 / % possible all: 99.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : Crystal 1 SINGLE WAVELENGTH PROTOCOL, Crysatl 2 MAD PROTOCOL : Crystal 1 SINGLE WAVELENGTH PROTOCOL, Crysatl 2 MAD PROTOCOLResolution: 2.4→38.11 Å / Rfactor Rfree error: 0.011 / Data cutoff high absF: 1353400.47 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 47.6302 Å2 / ksol: 0.329866 e/Å3 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 56.5 Å2
| ||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.4→38.11 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.4→2.55 Å / Rfactor Rfree error: 0.037 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj



