[English] 日本語
 Yorodumi
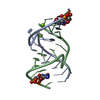
Yorodumi- PDB-1egr: SEQUENCE-SPECIFIC 1H N.M.R. ASSIGNMENTS AND DETERMINATION OF THE ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1egr | ||||||
|---|---|---|---|---|---|---|---|
| Title | SEQUENCE-SPECIFIC 1H N.M.R. ASSIGNMENTS AND DETERMINATION OF THE THREE-DIMENSIONAL STRUCTURE OF REDUCED ESCHERICHIA COLI GLUTAREDOXIN | ||||||
 Components Components | GLUTAREDOXIN | ||||||
 Keywords Keywords |  ELECTRON TRANSPORT ELECTRON TRANSPORT | ||||||
| Function / homology |  Function and homology information Function and homology informationcysteine biosynthetic process via S-sulfo-L-cysteine / sulfate assimilation via adenylyl sulfate reduction /  protein-disulfide reductase (glutathione) activity / glutathione disulfide oxidoreductase activity / disulfide oxidoreductase activity / deoxyribonucleotide biosynthetic process / protein-disulfide reductase (glutathione) activity / glutathione disulfide oxidoreductase activity / disulfide oxidoreductase activity / deoxyribonucleotide biosynthetic process /  protein-disulfide reductase activity / cell redox homeostasis / cellular response to oxidative stress / protein-disulfide reductase activity / cell redox homeostasis / cellular response to oxidative stress /  electron transfer activity ...cysteine biosynthetic process via S-sulfo-L-cysteine / sulfate assimilation via adenylyl sulfate reduction / electron transfer activity ...cysteine biosynthetic process via S-sulfo-L-cysteine / sulfate assimilation via adenylyl sulfate reduction /  protein-disulfide reductase (glutathione) activity / glutathione disulfide oxidoreductase activity / disulfide oxidoreductase activity / deoxyribonucleotide biosynthetic process / protein-disulfide reductase (glutathione) activity / glutathione disulfide oxidoreductase activity / disulfide oxidoreductase activity / deoxyribonucleotide biosynthetic process /  protein-disulfide reductase activity / cell redox homeostasis / cellular response to oxidative stress / protein-disulfide reductase activity / cell redox homeostasis / cellular response to oxidative stress /  electron transfer activity / electron transfer activity /  nucleotide binding / nucleotide binding /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli) | ||||||
| Method |  SOLUTION NMR SOLUTION NMR | ||||||
 Authors Authors | Sodano, P. / Xia, T.-H. / Bushweller, J.H. / Bjornberg, O. / Holmgren, A. / Billeter, M. / Wuthrich, K. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 1991 Journal: J.Mol.Biol. / Year: 1991Title: Sequence-specific 1H n.m.r. assignments and determination of the three-dimensional structure of reduced Escherichia coli glutaredoxin. Authors: Sodano, P. / Xia, T.H. / Bushweller, J.H. / Bjornberg, O. / Holmgren, A. / Billeter, M. / Wuthrich, K. #1:  Journal: Protein Sci. / Year: 1992 Journal: Protein Sci. / Year: 1992Title: The NMR Structure of Oxidized E. Coli Glutaredoxin. Comparison with Reduced E. Coli Glutaredoxin and Functionally Related Proteins Authors: Xia, T.-H. / Bushweller, J.H. / Sodano, P. / Billeter, M. / Bjornberg, O. / Holmgren, A. / Wuthrich, K. #2:  Journal: Eur.J.Biochem. / Year: 1991 Journal: Eur.J.Biochem. / Year: 1991Title: Nuclear Magnetic Resonance Studies of Recombinant Escherichia Coli Glutaredoxin: Sequence-Specific Assignments and Secondary Structure Determination for the Oxidized Form Authors: Sodano, P. / Chary, K.V.R. / Bjornberg, O. / Holmgren, A. / Kren, B. / Fuchs, J.A. / Wuthrich, K. #3:  Journal: Protein Expr.Purif. / Year: 1991 Journal: Protein Expr.Purif. / Year: 1991Title: Characterization of Homogeneous Recombinant Glutaredoxin from Escherichia Coli: Purification from an Inducible Lambda-P(L) Expression System and Properties of a Novel Elongated Form Protein ...Title: Characterization of Homogeneous Recombinant Glutaredoxin from Escherichia Coli: Purification from an Inducible Lambda-P(L) Expression System and Properties of a Novel Elongated Form Protein Expression and Purification Authors: Bjornberg, O. / Holmgren, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1egr.cif.gz 1egr.cif.gz | 580.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1egr.ent.gz pdb1egr.ent.gz | 495.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1egr.json.gz 1egr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/eg/1egr https://data.pdbj.org/pub/pdb/validation_reports/eg/1egr ftp://data.pdbj.org/pub/pdb/validation_reports/eg/1egr ftp://data.pdbj.org/pub/pdb/validation_reports/eg/1egr | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| Atom site foot note | 1: RESIDUE 60 IS A CIS PROLINE. | |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein |  Mass: 9695.823 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli (E. coli) / References: UniProt: P68688 Escherichia coli (E. coli) / References: UniProt: P68688 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR |
|---|
- Sample preparation
Sample preparation
Crystal grow | *PLUS Method: other / Details: NMR |
|---|
- Processing
Processing
| Software |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NMR software |
| |||||||||
| NMR ensemble | Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller











 PDBj
PDBj