[English] 日本語
 Yorodumi
Yorodumi- PDB-1afc: STRUCTURAL STUDIES OF THE BINDING OF THE ANTI-ULCER DRUG SUCROSE ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1afc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
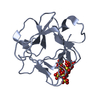
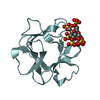
| Title | STRUCTURAL STUDIES OF THE BINDING OF THE ANTI-ULCER DRUG SUCROSE OCTASULFATE TO ACIDIC FIBROBLAST GROWTH FACTOR | |||||||||
 Components Components | ACIDIC FIBROBLAST GROWTH FACTOR FGF1 FGF1 | |||||||||
 Keywords Keywords |  GROWTH FACTOR GROWTH FACTOR | |||||||||
| Function / homology |  Function and homology information Function and homology informationFGFR4 ligand binding and activation / FGFR1b ligand binding and activation / FGFR3b ligand binding and activation / FGFR3c ligand binding and activation / FGFR1c ligand binding and activation / FGFR2c ligand binding and activation / FGFR2b ligand binding and activation / Phospholipase C-mediated cascade: FGFR1 / Phospholipase C-mediated cascade; FGFR2 / Phospholipase C-mediated cascade; FGFR3 ...FGFR4 ligand binding and activation / FGFR1b ligand binding and activation / FGFR3b ligand binding and activation / FGFR3c ligand binding and activation / FGFR1c ligand binding and activation / FGFR2c ligand binding and activation / FGFR2b ligand binding and activation / Phospholipase C-mediated cascade: FGFR1 / Phospholipase C-mediated cascade; FGFR2 / Phospholipase C-mediated cascade; FGFR3 / Phospholipase C-mediated cascade; FGFR4 / Downstream signaling of activated FGFR1 / SHC-mediated cascade:FGFR1 / FRS-mediated FGFR1 signaling / SHC-mediated cascade:FGFR2 / FRS-mediated FGFR2 signaling / SHC-mediated cascade:FGFR3 / FRS-mediated FGFR3 signaling / FRS-mediated FGFR4 signaling / SHC-mediated cascade:FGFR4 / RAF/MAP kinase cascade / PI-3K cascade:FGFR1 / PI-3K cascade:FGFR2 / PI-3K cascade:FGFR3 / PI-3K cascade:FGFR4 / PI3K Cascade / PIP3 activates AKT signaling / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / Negative regulation of FGFR1 signaling / Negative regulation of FGFR2 signaling / Negative regulation of FGFR3 signaling / Negative regulation of FGFR4 signaling / mesonephric epithelium development / branch elongation involved in ureteric bud branching / regulation of endothelial tube morphogenesis / positive regulation of cholesterol biosynthetic process /  fibroblast growth factor receptor binding / S100 protein binding / positive regulation of intracellular signal transduction / positive regulation of sprouting angiogenesis / positive regulation of cell division / fibroblast growth factor receptor signaling pathway / activation of protein kinase B activity / positive regulation of endothelial cell migration / positive regulation of epithelial cell proliferation / animal organ morphogenesis / fibroblast growth factor receptor binding / S100 protein binding / positive regulation of intracellular signal transduction / positive regulation of sprouting angiogenesis / positive regulation of cell division / fibroblast growth factor receptor signaling pathway / activation of protein kinase B activity / positive regulation of endothelial cell migration / positive regulation of epithelial cell proliferation / animal organ morphogenesis /  growth factor activity / lung development / growth factor activity / lung development /  wound healing / positive regulation of angiogenesis / wound healing / positive regulation of angiogenesis /  integrin binding / integrin binding /  heparin binding / cellular response to heat / heparin binding / cellular response to heat /  cell cortex / cell cortex /  angiogenesis / positive regulation of ERK1 and ERK2 cascade / angiogenesis / positive regulation of ERK1 and ERK2 cascade /  cell differentiation / positive regulation of cell migration / positive regulation of cell population proliferation / positive regulation of gene expression / positive regulation of transcription by RNA polymerase II / cell differentiation / positive regulation of cell migration / positive regulation of cell population proliferation / positive regulation of gene expression / positive regulation of transcription by RNA polymerase II /  extracellular space / extracellular region / extracellular space / extracellular region /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Bos taurus (cattle) Bos taurus (cattle) | |||||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.7 Å X-RAY DIFFRACTION / Resolution: 2.7 Å | |||||||||
 Authors Authors | Zhu, X. / Hsu, B.T. / Rees, D.C. | |||||||||
 Citation Citation |  Journal: Structure / Year: 1993 Journal: Structure / Year: 1993Title: Structural studies of the binding of the anti-ulcer drug sucrose octasulfate to acidic fibroblast growth factor. Authors: Zhu, X. / Hsu, B.T. / Rees, D.C. #1:  Journal: Science / Year: 1991 Journal: Science / Year: 1991Title: Three-Dimensional Structures of Acidic and Basic Fibroblast Growth Factors Authors: Zhu, X. / Komiya, H. / Chirino, A. / Faham, S. / Fox, G.M. / Arakawa, T. / Hsu, B.T. / Rees, D.C. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1afc.cif.gz 1afc.cif.gz | 216.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1afc.ent.gz pdb1afc.ent.gz | 171 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1afc.json.gz 1afc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/af/1afc https://data.pdbj.org/pub/pdb/validation_reports/af/1afc ftp://data.pdbj.org/pub/pdb/validation_reports/af/1afc ftp://data.pdbj.org/pub/pdb/validation_reports/af/1afc | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
- Components
Components
| #1: Protein |  FGF1 FGF1Mass: 15871.007 Da / Num. of mol.: 8 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Bos taurus (cattle) / Production host: Bos taurus (cattle) / Production host:   Escherichia coli (E. coli) / References: UniProt: P03968 Escherichia coli (E. coli) / References: UniProt: P03968#2: Polysaccharide | 1,3,4,6-tetra-O-sulfo-beta-D-fructofuranose-(2-1)-2,3,4,6-tetra-O-sulfonato-alpha-D-glucopyranose / sucrose octasulfate |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.39 Å3/Da / Density % sol: 48.61 % | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | *PLUS pH: 6.5 / Method: vapor diffusion, hanging drop | ||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Highest resolution: 2.7 Å / Num. obs: 31326 / % possible obs: 95 % / Num. measured all: 138212 / Rmerge(I) obs: 0.118 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Rfactor Rwork : 0.204 / Rfactor obs: 0.204 / Highest resolution: 2.7 Å : 0.204 / Rfactor obs: 0.204 / Highest resolution: 2.7 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 2.7 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.7 Å / Rfactor obs: 0.204 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: x_angle_d / Dev ideal: 2.7 |
 Movie
Movie Controller
Controller











 PDBj
PDBj