[English] 日本語
 Yorodumi
Yorodumi- EMDB-43293: Cryo-EM structure of Tulane virus 9-6-17 variant capsid protein V... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Tulane virus 9-6-17 variant capsid protein VP1 9-14-18 without DTT treatment | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | virion capsid /  capsid protein / capsid protein /  Tulane virus / Tulane virus /  VIRUS VIRUS | |||||||||
| Function / homology | Calicivirus coat protein C-terminal / Calicivirus coat protein C-terminal / Calicivirus coat protein / Calicivirus coat protein /  Viral coat protein subunit / Viral coat protein subunit /  Capsid protein Capsid protein Function and homology information Function and homology information | |||||||||
| Biological species |   Tulane virus Tulane virus | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.73 Å cryo EM / Resolution: 2.73 Å | |||||||||
 Authors Authors | Sun C / Jiang W | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Biomolecules / Year: 2024 Journal: Biomolecules / Year: 2024Title: The 2.6 Å Structure of a Tulane Virus Variant with Minor Mutations Leading to Receptor Change. Authors: Chen Sun / Pengwei Huang / Xueyong Xu / Frank S Vago / Kunpeng Li / Thomas Klose / Xi Jason Jiang / Wen Jiang /  Abstract: Human noroviruses (HuNoVs) are a major cause of acute gastroenteritis, contributing significantly to annual foodborne illness cases. However, studying these viruses has been challenging due to ...Human noroviruses (HuNoVs) are a major cause of acute gastroenteritis, contributing significantly to annual foodborne illness cases. However, studying these viruses has been challenging due to limitations in tissue culture techniques for over four decades. Tulane virus (TV) has emerged as a crucial surrogate for HuNoVs due to its close resemblance in amino acid composition and the availability of a robust cell culture system. Initially isolated from rhesus macaques in 2008, TV represents a novel belonging to the genus. Its significance lies in sharing the same host cell receptor, histo-blood group antigen (HBGA), as HuNoVs. In this study, we introduce, through cryo-electron microscopy (cryo-EM), the structure of a specific TV variant (the 9-6-17 TV) that has notably lost its ability to bind to its receptor, B-type HBGA-a finding confirmed using an enzyme-linked immunosorbent assay (ELISA). These results offer a profound insight into the genetic modifications occurring in TV that are necessary for adaptation to cell culture environments. This research significantly contributes to advancing our understanding of the genetic changes that are pivotal to successful adaptation, shedding light on fundamental aspects of evolution. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43293.map.gz emd_43293.map.gz | 314.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43293-v30.xml emd-43293-v30.xml emd-43293.xml emd-43293.xml | 15.1 KB 15.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_43293.png emd_43293.png | 250.6 KB | ||
| Masks |  emd_43293_msk_1.map emd_43293_msk_1.map emd_43293_msk_2.map emd_43293_msk_2.map | 824 MB 824 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43293.cif.gz emd-43293.cif.gz | 5.4 KB | ||
| Others |  emd_43293_half_map_1.map.gz emd_43293_half_map_1.map.gz emd_43293_half_map_2.map.gz emd_43293_half_map_2.map.gz | 313.9 MB 313.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43293 http://ftp.pdbj.org/pub/emdb/structures/EMD-43293 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43293 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43293 | HTTPS FTP |
-Related structure data
| Related structure data |  8vjsMC  8vgrC  8vjrC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43293.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43293.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.035 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43293_msk_1.map emd_43293_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
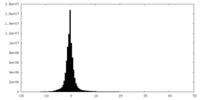
| Density Histograms |
-Mask #2
| File |  emd_43293_msk_2.map emd_43293_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
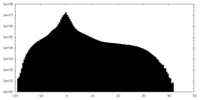
| Density Histograms |
-Half map: #2
| File | emd_43293_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_43293_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Tulane virus
| Entire | Name:   Tulane virus Tulane virus |
|---|---|
| Components |
|
-Supramolecule #1: Tulane virus
| Supramolecule | Name: Tulane virus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 512169 / Sci species name: Tulane virus / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:   Macaca mulatta (Rhesus monkey) Macaca mulatta (Rhesus monkey) |
| Molecular weight | Theoretical: 57 MDa |
| Virus shell | Shell ID: 1 / Name: Tulane virus / Diameter: 40.0 Å / T number (triangulation number): 3 |
-Macromolecule #1: Capsid protein
| Macromolecule | Name: Capsid protein / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Tulane virus Tulane virus |
| Molecular weight | Theoretical: 57.933172 KDa |
| Sequence | String: MESSKTEQVT GATGITQSTV TAPLPEAVSS LSLAPTVNAL DPWVYLNQTE VPGGTFTVSS ATQPGSVLLE LEISPELNLY TSHLFRMYA GWSGGFSLKL LVAGNAFSAG KLIAAIIPPN IEVPNSAYLL TGFPHEILDF RTADSMEIIA PDIKNIDYHF R GDKLGKLV ...String: MESSKTEQVT GATGITQSTV TAPLPEAVSS LSLAPTVNAL DPWVYLNQTE VPGGTFTVSS ATQPGSVLLE LEISPELNLY TSHLFRMYA GWSGGFSLKL LVAGNAFSAG KLIAAIIPPN IEVPNSAYLL TGFPHEILDF RTADSMEIIA PDIKNIDYHF R GDKLGKLV VMVYSPLRST SADFEIEIKL TSAPLPDFKF TMLVPPIQNN ALPIWSIPQA PPYSMVNPRS PLTPVVELYI NS SYATCNH QLGRYTIYQG AIGNSTFNPS GAWTATCTAE AGSVTGHPNW RYALLDLPDN PTFDPTLPPV PRGFCDWGSG VKS GNKQHL VCFTGKKVEG GFQDVDTHMW DYGDNETVGL DNTYQRTIYI KDPSLEKDAQ YLVIPMGVSG AANDDTVQVA PNCY GSWDY APTVAPPLGE QFVWFRSQLP ASKTTTTSGV NSVPVNVNAL MSPDLMCSAY ASGFPLGKVA LLDYVLFGGS VVRQF KLYP EGYMTANTTG SNTGFIIPAD GYFRFNSWVS PSFMISSVVD LNLQTAVVFR UniProtKB:  Capsid protein Capsid protein |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 25.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Initial angle assignment | Type: OTHER / Software - Name: cryoSPARC |
| Final angle assignment | Type: OTHER / Software - Name: jspr |
| Final reconstruction | Applied symmetry - Point group: I (icosahedral ) / Resolution.type: BY AUTHOR / Resolution: 2.73 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: jspr / Number images used: 16777 ) / Resolution.type: BY AUTHOR / Resolution: 2.73 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: jspr / Number images used: 16777 |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X