[English] 日本語
 Yorodumi
Yorodumi- EMDB-43160: Human transthyretin covalently modified with A2-derived stilbene ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human transthyretin covalently modified with A2-derived stilbene in the compressed conformation | |||||||||
 Map data Map data | Compressed A2 TTR map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Amyloidosis / Amyloidosis /  TRANSPORT PROTEIN TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationRetinoid cycle disease events /  thyroid hormone binding / The canonical retinoid cycle in rods (twilight vision) / Non-integrin membrane-ECM interactions / purine nucleobase metabolic process / Retinoid metabolism and transport / thyroid hormone binding / The canonical retinoid cycle in rods (twilight vision) / Non-integrin membrane-ECM interactions / purine nucleobase metabolic process / Retinoid metabolism and transport /  hormone activity / azurophil granule lumen / Amyloid fiber formation / Neutrophil degranulation ...Retinoid cycle disease events / hormone activity / azurophil granule lumen / Amyloid fiber formation / Neutrophil degranulation ...Retinoid cycle disease events /  thyroid hormone binding / The canonical retinoid cycle in rods (twilight vision) / Non-integrin membrane-ECM interactions / purine nucleobase metabolic process / Retinoid metabolism and transport / thyroid hormone binding / The canonical retinoid cycle in rods (twilight vision) / Non-integrin membrane-ECM interactions / purine nucleobase metabolic process / Retinoid metabolism and transport /  hormone activity / azurophil granule lumen / Amyloid fiber formation / Neutrophil degranulation / hormone activity / azurophil granule lumen / Amyloid fiber formation / Neutrophil degranulation /  extracellular space / extracellular exosome / extracellular region / identical protein binding extracellular space / extracellular exosome / extracellular region / identical protein bindingSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.1 Å cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Basanta B / Nugroho K / Yan N / Kline GM / Tsai FJ / Wu M / Kelly JW / Lander GC | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: The conformational landscape of human transthyretin revealed by cryo-EM Authors: Basanta B / Nugroho K / Yan N / Kline GM / Powers ET / Tsai FJ / Wu M / Hansel-Harris A / Chen J / Forli S / Kelly JW / Lander GC | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43160.map.gz emd_43160.map.gz | 10.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43160-v30.xml emd-43160-v30.xml emd-43160.xml emd-43160.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_43160.png emd_43160.png | 53.6 KB | ||
| Masks |  emd_43160_msk_1.map emd_43160_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43160.cif.gz emd-43160.cif.gz | 6.6 KB | ||
| Others |  emd_43160_half_map_1.map.gz emd_43160_half_map_1.map.gz emd_43160_half_map_2.map.gz emd_43160_half_map_2.map.gz | 49.7 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43160 http://ftp.pdbj.org/pub/emdb/structures/EMD-43160 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43160 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43160 | HTTPS FTP |
-Related structure data
| Related structure data |  8ve0MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43160.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43160.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Compressed A2 TTR map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.562 Å | ||||||||||||||||||||
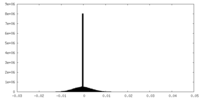
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43160_msk_1.map emd_43160_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
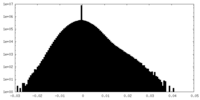
| Density Histograms |
-Half map: Compressed A2 TTR half map 1
| File | emd_43160_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Compressed A2 TTR half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Compressed A2 TTR half map 2
| File | emd_43160_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Compressed A2 TTR half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Transthyretin
| Entire | Name: Transthyretin |
|---|---|
| Components |
|
-Supramolecule #1: Transthyretin
| Supramolecule | Name: Transthyretin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56.136 KDa |
-Macromolecule #1: Transthyretin
| Macromolecule | Name: Transthyretin / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.908557 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MGPTGTGESK CPLMVKVLDA VRGSPAINVA VHVFRKAADD TWEPFASGKT SESGELHGLT TEEEFVEGIY KVEIDTKSYW KALGISPFH EHAEVVFTAN DSGPRRYTIA ALLSPYSYST TAVVTNPKE UniProtKB:  Transthyretin Transthyretin |
-Macromolecule #2: 3-(dimethylamino)-5-[(E)-2-(4-hydroxy-3,5-dimethylphenyl)ethenyl]...
| Macromolecule | Name: 3-(dimethylamino)-5-[(E)-2-(4-hydroxy-3,5-dimethylphenyl)ethenyl]benzoic acid type: ligand / ID: 2 / Number of copies: 2 / Formula: 1WZ |
|---|---|
| Molecular weight | Theoretical: 311.375 Da |
| Chemical component information | 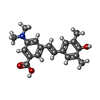 ChemComp-1WZ: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.41 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.6 Component:
| |||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE / Support film - topology: CONTINUOUS / Pretreatment - Time: 240 sec. / Pretreatment - Atmosphere: OTHER Details: Graphene grids were treated with UV/ozone using the UVOCS T10xT10 system. A 10-minute warmup run was performed immediately prior to inserting and treating the grids for 4 minutes. | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 279 K / Instrument: HOMEMADE PLUNGER Details: 3 uL of sample were applied onto the graphene side of the grid after it was mounted on the plunger (always at the same height), and immediately blotted for 6 seconds by holding a 1 x 6 cm ...Details: 3 uL of sample were applied onto the graphene side of the grid after it was mounted on the plunger (always at the same height), and immediately blotted for 6 seconds by holding a 1 x 6 cm piece of Whatman 1 filter paper parallel to the grid, in full contact. Timing was kept with the help of a metronome. The blotting countdown was started after the blotted liquid spot on the filter paper stopped spreading and the grid was plunged in liquid ethane at the same time the blotting paper was pulled back, in a single motion.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 1.5 µm / Calibrated defocus min: 0.5 µm / Calibrated magnification: 88968 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 73000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 73000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Temperature | Min: 78.0 K / Max: 85.0 K |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Number grids imaged: 1 / Number real images: 4536 / Average exposure time: 6.8 sec. / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.1 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.1) / Number images used: 105961 |
 Movie
Movie Controller
Controller








 Z
Z Y
Y X
X