[English] 日本語
 Yorodumi
Yorodumi- EMDB-41043: Structure of mechanically activated ion channel OSCA1.2 in peptidiscs -
+ Open data
Open data
- Basic information
Basic information
| Entry | 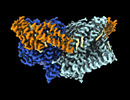 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of mechanically activated ion channel OSCA1.2 in peptidiscs | |||||||||
 Map data Map data | OSCA1.2 in peptidisc postprocessed with LocalDeblur | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | mechanically activated ion channel /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmechanosensitive monoatomic ion channel activity / calcium-activated cation channel activity / monoatomic cation transport / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Arabidopsis thaliana (thale cress) / synthetic construct (others) Arabidopsis thaliana (thale cress) / synthetic construct (others) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.8 Å cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Burendei B / Jojoa-Cruz S / Lee WH / Ward AB | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: Structure of mechanically activated ion channel OSCA2.3 reveals mobile elements in the transmembrane domain. Authors: Sebastian Jojoa-Cruz / Batuujin Burendei / Wen-Hsin Lee / Andrew B Ward /  Abstract: Members of the OSCA/TMEM63 family are mechanically activated ion channels and structures of some OSCA members have revealed the architecture of these channels and structural features that are ...Members of the OSCA/TMEM63 family are mechanically activated ion channels and structures of some OSCA members have revealed the architecture of these channels and structural features that are potentially involved in mechanosensation. However, these structures are all in a similar state and information about the motion of different elements of the structure is limited, preventing a deeper understanding of how these channels work. Here, we used cryoelectron microscopy to determine high-resolution structures of Arabidopsis thaliana OSCA1.2 and OSCA2.3 in peptidiscs. The structure of OSCA1.2 matches previous structures of the same protein in different environments. Yet, in OSCA2.3, the TM6a-TM7 linker adopts a different conformation that constricts the pore on its cytoplasmic side. Furthermore, coevolutionary sequence analysis uncovered a conserved interaction between the TM6a-TM7 linker and the beam-like domain (BLD). Our results reveal conformational heterogeneity and differences in conserved interactions between the TMD and BLD among members of the OSCA family. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41043.map.gz emd_41043.map.gz | 30.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41043-v30.xml emd-41043-v30.xml emd-41043.xml emd-41043.xml | 24 KB 24 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41043_fsc.xml emd_41043_fsc.xml | 7.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_41043.png emd_41043.png | 111 KB | ||
| Filedesc metadata |  emd-41043.cif.gz emd-41043.cif.gz | 7.1 KB | ||
| Others |  emd_41043_additional_1.map.gz emd_41043_additional_1.map.gz emd_41043_half_map_1.map.gz emd_41043_half_map_1.map.gz emd_41043_half_map_2.map.gz emd_41043_half_map_2.map.gz | 35.2 MB 30.4 MB 30.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41043 http://ftp.pdbj.org/pub/emdb/structures/EMD-41043 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41043 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41043 | HTTPS FTP |
-Related structure data
| Related structure data |  8t56MC  8t57C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41043.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41043.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | OSCA1.2 in peptidisc postprocessed with LocalDeblur | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: OSCA1.2 in peptidisc postprocessed with DeepEMhancer "highRes" model
| File | emd_41043_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | OSCA1.2 in peptidisc postprocessed with DeepEMhancer "highRes" model | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: OSCA1.2 in peptidisc half map 2
| File | emd_41043_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | OSCA1.2 in peptidisc half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: OSCA1.2 in peptidisc half map 1
| File | emd_41043_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | OSCA1.2 in peptidisc half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : OSCA1.2 in peptidisc
| Entire | Name: OSCA1.2 in peptidisc |
|---|---|
| Components |
|
-Supramolecule #1: OSCA1.2 in peptidisc
| Supramolecule | Name: OSCA1.2 in peptidisc / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Molecular weight | Theoretical: 176 KDa |
-Supramolecule #2: OSCA1.2
| Supramolecule | Name: OSCA1.2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Arabidopsis thaliana (thale cress) Arabidopsis thaliana (thale cress) |
-Supramolecule #3: peptidisc peptide
| Supramolecule | Name: peptidisc peptide / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) / Synthetically produced: Yes |
-Macromolecule #1: Calcium permeable stress-gated cation channel 1
| Macromolecule | Name: Calcium permeable stress-gated cation channel 1 / type: protein_or_peptide / ID: 1 Details: The last 10 residues (GTGTLEVLFQ) are leftover of a linker and protease site. Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Arabidopsis thaliana (thale cress) Arabidopsis thaliana (thale cress) |
| Molecular weight | Theoretical: 89.031719 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MATLQDIGVS AGINILSAFV FFIIFAVLRL QPFNDRVYFS KWYLKGLRSS PARGGAFAQR FVNLDFRSYM KFLNWMPEAL KMPEPELID HAGLDSVVYL RIYWLGLKIF TPIAVLAWAV LVPVNWTNNT LEMAKQLRNV TSSDIDKLSV SNIPEYSMRF W THIVMAYA ...String: MATLQDIGVS AGINILSAFV FFIIFAVLRL QPFNDRVYFS KWYLKGLRSS PARGGAFAQR FVNLDFRSYM KFLNWMPEAL KMPEPELID HAGLDSVVYL RIYWLGLKIF TPIAVLAWAV LVPVNWTNNT LEMAKQLRNV TSSDIDKLSV SNIPEYSMRF W THIVMAYA FTIWTCYVLM KEYETIANMR LQFVASEARR PDQFTVLVRN VPPDADESVS ELVEHFFLVN HPDHYLTHQV VC NANKLAD LVKKKKKLQN WLDYYQLKYA RNNSQRIMVK LGFLGLWGQK VDAIEHYIAE IDKISKEISK EREEVVNDPK AIM PAAFVS FKTRWAAAVC AQTQQTRNPT QWLTEWAPEP RDVFWSNLAI PYVSLTVRRL IMHVAFFFLT FFFIVPIAFV QSLA TIEGI VKAAPFLKFI VDDKFMKSVI QGFLPGIALK LFLAFLPSIL MIMSKFEGFT SISSLERRAA FRYYIFNLVN VFLAS VIAG AAFEQLNSFL NQSANQIPKT IGVAIPMKAT FFITYIMVDG WAGVAGEILM LKPLIMFHLK NAFLVKTDKD REEAMD PGS IGFNTGEPRI QLYFLLGLVY APVTPMLLPF ILVFFALAYI VYRHQIINVY NQEYESAAAF WPDVHGRVIA ALVISQL LL MGLLGTKHAA LAAPFLIALP VLTIGFHHFC KGRYEPAFIR YPLQEAMMKD TLETAREPNL NLKGYLQNAY VHPVFKGD E DDYDIDDKLG KFEDEAIIVP TKRQSRRNTP APSIISGDDS PSLPFSGKLV GTGTLEVLFQ UniProtKB: Calcium permeable stress-gated cation channel 1 |
-Macromolecule #2: NSPr peptide
| Macromolecule | Name: NSPr peptide / type: protein_or_peptide / ID: 2 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 4.482132 KDa |
| Sequence | String: FAEKFKEAVK DYFAKFWDPA AEKLKEAVKD YFAKLWD |
-Macromolecule #3: CHOLESTEROL HEMISUCCINATE
| Macromolecule | Name: CHOLESTEROL HEMISUCCINATE / type: ligand / ID: 3 / Number of copies: 2 / Formula: Y01 |
|---|---|
| Molecular weight | Theoretical: 486.726 Da |
| Chemical component information |  ChemComp-Y01: |
-Macromolecule #4: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 4 / Number of copies: 26 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Macromolecule #5: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
| Macromolecule | Name: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine / type: ligand / ID: 5 / Number of copies: 2 / Formula: LBN |
|---|---|
| Molecular weight | Theoretical: 760.076 Da |
| Chemical component information |  ChemComp-LBN: |
-Macromolecule #6: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 6 / Number of copies: 6 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Material: GOLD / Mesh: 300 | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.4000000000000001 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 29000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 1.4000000000000001 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3710 pixel / Digitization - Dimensions - Height: 3838 pixel / Number grids imaged: 1 / Number real images: 1805 / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z
Z Y
Y X
X



























