+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli dodecamer SIR2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  SIR2 / NADase / SIR2 / NADase /  Nuclease / Anti-phage system / Nuclease / Anti-phage system /  IMMUNE SYSTEM IMMUNE SYSTEM | |||||||||
| Function / homology | SIR2-like domain / SIR2-like domain / SIR2-like domain-containing protein Function and homology information Function and homology information | |||||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||
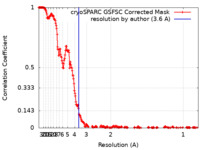
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Shen ZF / Lin QP / Fu TM | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: Assembly-mediated activation of the SIR2-HerA supramolecular complex for anti-phage defense. Authors: Zhangfei Shen / Qingpeng Lin / Xiao-Yuan Yang / Elizabeth Fosuah / Tian-Min Fu /  Abstract: SIR2-HerA, a bacterial two-protein anti-phage defense system, induces bacterial death by depleting NAD upon phage infection. Biochemical reconstitution of SIR2, HerA, and the SIR2-HerA complex ...SIR2-HerA, a bacterial two-protein anti-phage defense system, induces bacterial death by depleting NAD upon phage infection. Biochemical reconstitution of SIR2, HerA, and the SIR2-HerA complex reveals a dynamic assembly process. Unlike other ATPases, HerA can form various oligomers, ranging from dimers to nonamers. When assembled with SIR2, HerA forms a hexamer and converts SIR2 from a nuclease to an NAD hydrolase, representing an unexpected regulatory mechanism mediated by protein assembly. Furthermore, high concentrations of ATP can inhibit NAD hydrolysis by the SIR2-HerA complex. Cryo-EM structures of the SIR2-HerA complex reveal a giant supramolecular assembly up to 1 MDa, with SIR2 as a dodecamer and HerA as a hexamer, crucial for anti-phage defense. Unexpectedly, the HerA hexamer resembles a spiral staircase and exhibits helicase activities toward dual-forked DNA. Together, we reveal the supramolecular assembly of SIR2-HerA as a unique mechanism for switching enzymatic activities and bolstering anti-phage defense strategies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40860.map.gz emd_40860.map.gz | 1.3 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40860-v30.xml emd-40860-v30.xml emd-40860.xml emd-40860.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40860_fsc.xml emd_40860_fsc.xml | 24.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_40860.png emd_40860.png | 71.9 KB | ||
| Filedesc metadata |  emd-40860.cif.gz emd-40860.cif.gz | 5.4 KB | ||
| Others |  emd_40860_additional_1.map.gz emd_40860_additional_1.map.gz emd_40860_half_map_1.map.gz emd_40860_half_map_1.map.gz emd_40860_half_map_2.map.gz emd_40860_half_map_2.map.gz | 1.2 GB 1.3 GB 1.3 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40860 http://ftp.pdbj.org/pub/emdb/structures/EMD-40860 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40860 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40860 | HTTPS FTP |
-Related structure data
| Related structure data |  8sxxMC  8su9C  8subC  8suwC  8uaeC  8uafC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40860.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40860.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.4495 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_40860_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_40860_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_40860_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : E. coli dodecamer SIR2
| Entire | Name: E. coli dodecamer SIR2 |
|---|---|
| Components |
|
-Supramolecule #1: E. coli dodecamer SIR2
| Supramolecule | Name: E. coli dodecamer SIR2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 / Details: Six dimeric SIR2 form a dodecamer |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Macromolecule #1: SIR2-like domain-containing protein
| Macromolecule | Name: SIR2-like domain-containing protein / type: protein_or_peptide / ID: 1 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 46.817664 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MSIYQGGNKL NEDDFRSHVY SLCQLDNVGV LLGAGASVGC GGKTMKDVWK SFKQNYPELL GALIDKYLLV SQIDSDNNLV NVELLIDEA TKFLSVAKTR RCEDEEEEFR KILSSLYKEV TKAALLTGEQ FREKNQGKKD AFKYHKELIS KLISNRQPGQ S APAIFTTN ...String: MSIYQGGNKL NEDDFRSHVY SLCQLDNVGV LLGAGASVGC GGKTMKDVWK SFKQNYPELL GALIDKYLLV SQIDSDNNLV NVELLIDEA TKFLSVAKTR RCEDEEEEFR KILSSLYKEV TKAALLTGEQ FREKNQGKKD AFKYHKELIS KLISNRQPGQ S APAIFTTN YDLALEWAAE DLGIQLFNGF SGLHTRQFYP QNFDLAFRNV NAKGEARFGH YHAYLYKLHG SLTWYQNDSL TV NEVSASQ AYDEYINDII NKDDFYRGQH LIYPGANKYS HTIGFVYGEM FRRFGEFISK PQTALFINGF GFGDYHINRI ILG ALLNPS FHVVIYYPEL KEAITKVSKG GGSEAEKAIV TLKNMAFNQV TVVGGGSKAY FNSFVEHLPY PVLFPRDNIV DELV EAIAN LSKGEGNVPF UniProtKB: SIR2-like domain-containing protein |
-Macromolecule #2: NICOTINAMIDE-ADENINE-DINUCLEOTIDE
| Macromolecule | Name: NICOTINAMIDE-ADENINE-DINUCLEOTIDE / type: ligand / ID: 2 / Number of copies: 12 / Formula: NAD |
|---|---|
| Molecular weight | Theoretical: 663.425 Da |
| Chemical component information |  ChemComp-NAD: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z
Z Y
Y X
X