[English] 日本語
 Yorodumi
Yorodumi- EMDB-33992: Cryo-EM structure of EBV gHgL-gp42 in complex with mAb 10E4 (loca... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 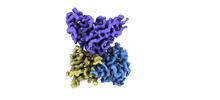 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of EBV gHgL-gp42 in complex with mAb 10E4 (localized refinement) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | EBV /  Cryo-EM / Cryo-EM /  Glycoprotein / gHgL-gp42 complex / Glycoprotein / gHgL-gp42 complex /  Antibody / Antibody /  VIRAL PROTEIN VIRAL PROTEIN | |||||||||
| Biological species |   Human gammaherpesvirus 4 (Epstein-Barr virus) / Human gammaherpesvirus 4 (Epstein-Barr virus) /   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.54 Å cryo EM / Resolution: 3.54 Å | |||||||||
 Authors Authors | Liu L / Sun H / Jiang Y / Hong J / Zheng Q / Li S / Chen Y / Xia N | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep Med / Year: 2023 Journal: Cell Rep Med / Year: 2023Title: Non-overlapping epitopes on the gHgL-gp42 complex for the rational design of a triple-antibody cocktail against EBV infection. Authors: Junping Hong / Ling Zhong / Liqin Liu / Qian Wu / Wanlin Zhang / Kaiyun Chen / Dongmei Wei / Hui Sun / Xiang Zhou / Xinyu Zhang / Yin-Feng Kang / Yang Huang / Junyu Chen / Guosong Wang / Yan ...Authors: Junping Hong / Ling Zhong / Liqin Liu / Qian Wu / Wanlin Zhang / Kaiyun Chen / Dongmei Wei / Hui Sun / Xiang Zhou / Xinyu Zhang / Yin-Feng Kang / Yang Huang / Junyu Chen / Guosong Wang / Yan Zhou / Yanhong Chen / Qi-Sheng Feng / Hai Yu / Shaowei Li / Mu-Sheng Zeng / Yi-Xin Zeng / Miao Xu / Qingbing Zheng / Yixin Chen / Xiao Zhang / Ningshao Xia /   Abstract: Epstein-Barr virus (EBV) is closely associated with cancer, multiple sclerosis, and post-acute coronavirus disease 2019 (COVID-19) sequelae. There are currently no approved therapeutics or vaccines ...Epstein-Barr virus (EBV) is closely associated with cancer, multiple sclerosis, and post-acute coronavirus disease 2019 (COVID-19) sequelae. There are currently no approved therapeutics or vaccines against EBV. It is noteworthy that combining multiple EBV glycoproteins can elicit potent neutralizing antibodies (nAbs) against viral infection, suggesting possible synergistic effects. Here, we characterize three nAbs (anti-gp42 5E3, anti-gHgL 6H2, and anti-gHgL 10E4) targeting different glycoproteins of the gHgL-gp42 complex. Two antibody cocktails synergistically neutralize infection in B cells (5E3+6H2+10E4) and epithelial cells (6H2+10E4) in vitro. Moreover, 5E3 alone and the 5E3+6H2+10E4 cocktail confer potent in vivo protection against lethal EBV challenge in humanized mice. The cryo-EM structure of a heptatomic gHgL-gp42 immune complex reveals non-overlapping epitopes of 5E3, 6H2, and 10E4 on the gHgL-gp42 complex. Structural and functional analyses highlight different neutralization mechanisms for each of the three nAbs. In summary, our results provide insight for the rational design of therapeutics or vaccines against EBV infection. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33992.map.gz emd_33992.map.gz | 323.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33992-v30.xml emd-33992-v30.xml emd-33992.xml emd-33992.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33992.png emd_33992.png | 32.9 KB | ||
| Filedesc metadata |  emd-33992.cif.gz emd-33992.cif.gz | 5.5 KB | ||
| Others |  emd_33992_half_map_1.map.gz emd_33992_half_map_1.map.gz emd_33992_half_map_2.map.gz emd_33992_half_map_2.map.gz | 318.7 MB 318.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33992 http://ftp.pdbj.org/pub/emdb/structures/EMD-33992 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33992 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33992 | HTTPS FTP |
-Related structure data
| Related structure data |  7yp1MC  7yoyC  7yp2C M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_33992.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33992.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.778 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_33992_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_33992_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : gHgL-gp42 in complex with mAb 10E4
| Entire | Name: gHgL-gp42 in complex with mAb 10E4 |
|---|---|
| Components |
|
-Supramolecule #1: gHgL-gp42 in complex with mAb 10E4
| Supramolecule | Name: gHgL-gp42 in complex with mAb 10E4 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: gH,gL
| Supramolecule | Name: gH,gL / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Human gammaherpesvirus 4 (Epstein-Barr virus) Human gammaherpesvirus 4 (Epstein-Barr virus) |
-Supramolecule #3: 10E4
| Supramolecule | Name: 100000 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3-#4 |
|---|---|
| Source (natural) | Organism:   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
-Macromolecule #1: EBV gH
| Macromolecule | Name: EBV gH / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human gammaherpesvirus 4 (Epstein-Barr virus) Human gammaherpesvirus 4 (Epstein-Barr virus) |
| Molecular weight | Theoretical: 22.774117 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: LSEVKLHLDI EGHASHYTIP WTELMAKVPG LSPEALWREA NVTEDLASML NRYKLIYKTS GTLGIALASA PLEKQLFYYI GTMLPNTRP HSYVFYQLRC HLSYVALSIN GDKFQYTGAM TSKFLMGTYK RVTEKGDEHV LSLVFGKTKD LPDLRGPFSY P SLTSAQSG ...String: LSEVKLHLDI EGHASHYTIP WTELMAKVPG LSPEALWREA NVTEDLASML NRYKLIYKTS GTLGIALASA PLEKQLFYYI GTMLPNTRP HSYVFYQLRC HLSYVALSIN GDKFQYTGAM TSKFLMGTYK RVTEKGDEHV LSLVFGKTKD LPDLRGPFSY P SLTSAQSG DYSLVIVTTF VHYANFHNYF VPNLKDMFSR AVT |
-Macromolecule #2: EBV gL
| Macromolecule | Name: EBV gL / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human gammaherpesvirus 4 (Epstein-Barr virus) Human gammaherpesvirus 4 (Epstein-Barr virus) |
| Molecular weight | Theoretical: 8.623719 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: LALENISDIY LVSNQTCDGF SLASLNSVIS RCANGLNVVS FFISILKRSS SALTGHLREL LTTLETLYGS FSVEDLFGAN |
-Macromolecule #3: 10E4 heavy chain
| Macromolecule | Name: 10E4 heavy chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| Molecular weight | Theoretical: 12.273763 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QQVKESGGRL VTPGTPLTLT CTASGFSLST YWMSWVRQAP GKGLEYIGVI GGSGSTYYAS WAKGRFTISK TSTTVDLKIT SPTTEDTAT YFCARDSGAG VRFRFWGPGT LVTVSS |
-Macromolecule #4: 10E4 light chain
| Macromolecule | Name: 10E4 light chain / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Oryctolagus cuniculus (rabbit) Oryctolagus cuniculus (rabbit) |
| Molecular weight | Theoretical: 11.917251 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DLVMTQTPAS VEAGVGGTVT INCQASENIG SRLAWYQQKP GQPPKLLIYR ASTLESGVPS RFKGSGSGTE FTLTISDLEC ADAATYYCQ CTYGVSITIN YGNDFGGGTE VVVK |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.54 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 402382 |
 Movie
Movie Controller
Controller




 Z
Z Y
Y X
X