+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
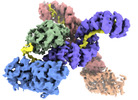
| Title | Human O-GlcNAc transferase Dimer | |||||||||
 Map data Map data | Human OGT Dimer | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Human O-GlcNAc transferase Dimer /  TRANSFERASE TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology information protein N-acetylglucosaminyltransferase complex / protein N-acetylglucosaminyltransferase complex /  protein O-GlcNAc transferase / regulation of insulin receptor signaling pathway / protein O-acetylglucosaminyltransferase activity / positive regulation of transcription from RNA polymerase II promoter by glucose / acetylglucosaminyltransferase activity / regulation of necroptotic process / regulation of Rac protein signal transduction / negative regulation of stem cell population maintenance / protein O-linked glycosylation ... protein O-GlcNAc transferase / regulation of insulin receptor signaling pathway / protein O-acetylglucosaminyltransferase activity / positive regulation of transcription from RNA polymerase II promoter by glucose / acetylglucosaminyltransferase activity / regulation of necroptotic process / regulation of Rac protein signal transduction / negative regulation of stem cell population maintenance / protein O-linked glycosylation ... protein N-acetylglucosaminyltransferase complex / protein N-acetylglucosaminyltransferase complex /  protein O-GlcNAc transferase / regulation of insulin receptor signaling pathway / protein O-acetylglucosaminyltransferase activity / positive regulation of transcription from RNA polymerase II promoter by glucose / acetylglucosaminyltransferase activity / regulation of necroptotic process / regulation of Rac protein signal transduction / negative regulation of stem cell population maintenance / protein O-linked glycosylation / NSL complex / regulation of glycolytic process / regulation of neurotransmitter receptor localization to postsynaptic specialization membrane / RIPK1-mediated regulated necrosis / protein O-GlcNAc transferase / regulation of insulin receptor signaling pathway / protein O-acetylglucosaminyltransferase activity / positive regulation of transcription from RNA polymerase II promoter by glucose / acetylglucosaminyltransferase activity / regulation of necroptotic process / regulation of Rac protein signal transduction / negative regulation of stem cell population maintenance / protein O-linked glycosylation / NSL complex / regulation of glycolytic process / regulation of neurotransmitter receptor localization to postsynaptic specialization membrane / RIPK1-mediated regulated necrosis /  regulation of synapse assembly / regulation of synapse assembly /  regulation of gluconeogenesis / positive regulation of stem cell population maintenance / Formation of WDR5-containing histone-modifying complexes / phosphatidylinositol-3,4,5-trisphosphate binding / positive regulation of proteolysis / regulation of gluconeogenesis / positive regulation of stem cell population maintenance / Formation of WDR5-containing histone-modifying complexes / phosphatidylinositol-3,4,5-trisphosphate binding / positive regulation of proteolysis /  mitophagy / mitophagy /  hemopoiesis / negative regulation of proteasomal ubiquitin-dependent protein catabolic process / hemopoiesis / negative regulation of proteasomal ubiquitin-dependent protein catabolic process /  histone acetyltransferase complex / positive regulation of lipid biosynthetic process / negative regulation of protein ubiquitination / positive regulation of TORC1 signaling / negative regulation of cell migration / response to nutrient / cell projection / positive regulation of translation / histone acetyltransferase complex / positive regulation of lipid biosynthetic process / negative regulation of protein ubiquitination / positive regulation of TORC1 signaling / negative regulation of cell migration / response to nutrient / cell projection / positive regulation of translation /  mitochondrial membrane / cellular response to glucose stimulus / negative regulation of transforming growth factor beta receptor signaling pathway / circadian regulation of gene expression / response to insulin / Regulation of necroptotic cell death / protein processing / chromatin DNA binding / UCH proteinases / positive regulation of cold-induced thermogenesis / chromatin organization / HATs acetylate histones / glutamatergic synapse / apoptotic process / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / mitochondrial membrane / cellular response to glucose stimulus / negative regulation of transforming growth factor beta receptor signaling pathway / circadian regulation of gene expression / response to insulin / Regulation of necroptotic cell death / protein processing / chromatin DNA binding / UCH proteinases / positive regulation of cold-induced thermogenesis / chromatin organization / HATs acetylate histones / glutamatergic synapse / apoptotic process / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II /  signal transduction / positive regulation of transcription by RNA polymerase II / protein-containing complex / signal transduction / positive regulation of transcription by RNA polymerase II / protein-containing complex /  nucleoplasm / nucleoplasm /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.82 Å cryo EM / Resolution: 3.82 Å | |||||||||
 Authors Authors | Gao H / Lu P / Liu Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Cryo-EM structure of human O-GlcNAcylation enzyme pair OGT-OGA complex. Authors: Ping Lu / Yusong Liu / Maozhou He / Ting Cao / Mengquan Yang / Shutao Qi / Hongtao Yu / Haishan Gao /  Abstract: O-GlcNAcylation is a conserved post-translational modification that attaches N-acetyl glucosamine (GlcNAc) to myriad cellular proteins. In response to nutritional and hormonal signals, O- ...O-GlcNAcylation is a conserved post-translational modification that attaches N-acetyl glucosamine (GlcNAc) to myriad cellular proteins. In response to nutritional and hormonal signals, O-GlcNAcylation regulates diverse cellular processes by modulating the stability, structure, and function of target proteins. Dysregulation of O-GlcNAcylation has been implicated in the pathogenesis of cancer, diabetes, and neurodegeneration. A single pair of enzymes, the O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), catalyzes the addition and removal of O-GlcNAc on over 3,000 proteins in the human proteome. However, how OGT selects its native substrates and maintains the homeostatic control of O-GlcNAcylation of so many substrates against OGA is not fully understood. Here, we present the cryo-electron microscopy (cryo-EM) structures of human OGT and the OGT-OGA complex. Our studies reveal that OGT forms a functionally important scissor-shaped dimer. Within the OGT-OGA complex structure, a long flexible OGA segment occupies the extended substrate-binding groove of OGT and positions a serine for O-GlcNAcylation, thus preventing OGT from modifying other substrates. Conversely, OGT disrupts the functional dimerization of OGA and occludes its active site, resulting in the blocking of access by other substrates. This mutual inhibition between OGT and OGA may limit the futile O-GlcNAcylation cycles and help to maintain O-GlcNAc homeostasis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33768.map.gz emd_33768.map.gz | 229.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33768-v30.xml emd-33768-v30.xml emd-33768.xml emd-33768.xml | 17.9 KB 17.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33768.png emd_33768.png | 103.4 KB | ||
| Filedesc metadata |  emd-33768.cif.gz emd-33768.cif.gz | 6.3 KB | ||
| Others |  emd_33768_half_map_1.map.gz emd_33768_half_map_1.map.gz emd_33768_half_map_2.map.gz emd_33768_half_map_2.map.gz | 226.1 MB 226 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33768 http://ftp.pdbj.org/pub/emdb/structures/EMD-33768 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33768 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33768 | HTTPS FTP |
-Related structure data
| Related structure data |  7yeaMC  7yehC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33768.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33768.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Human OGT Dimer | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.861 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half map
| File | emd_33768_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map
| File | emd_33768_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human O-GlcNAc transferase (OGT)
| Entire | Name: Human O-GlcNAc transferase (OGT) |
|---|---|
| Components |
|
-Supramolecule #1: Human O-GlcNAc transferase (OGT)
| Supramolecule | Name: Human O-GlcNAc transferase (OGT) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 250 KDa |
-Macromolecule #1: UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase ...
| Macromolecule | Name: UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number:  protein O-GlcNAc transferase protein O-GlcNAc transferase |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 117.937367 KDa |
| Recombinant expression | Organism:   Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) |
| Sequence | String: MASSVGNVAD STEPTKRMLS FQGPMELAHR EYQAGDFEAA ERHCMQLWRQ EPDNTGVLLL LSSIHFECRR LDRSAHFSTL AIKQNPLLA EAYSNLGNVY KERGQLQEAI EHYRHALRLK PDFIDGYINL AAALVAAGDM EGAVQAYVSA LQYNPDLYCV R SDLGNLLK ...String: MASSVGNVAD STEPTKRMLS FQGPMELAHR EYQAGDFEAA ERHCMQLWRQ EPDNTGVLLL LSSIHFECRR LDRSAHFSTL AIKQNPLLA EAYSNLGNVY KERGQLQEAI EHYRHALRLK PDFIDGYINL AAALVAAGDM EGAVQAYVSA LQYNPDLYCV R SDLGNLLK ALGRLEEAKA CYLKAIETQP NFAVAWSNLG CVFNAQGEIW LAIHHFEKAV TLDPNFLDAY INLGNVLKEA RI FDRAVAA YLRALSLSPN HAVVHGNLAC VYYEQGLIDL AIDTYRRAIE LQPHFPDAYC NLANALKEKG SVAEAEDCYN TAL RLCPTH ADSLNNLANI KREQGNIEEA VRLYRKALEV FPEFAAAHSN LASVLQQQGK LQEALMHYKE AIRISPTFAD AYSN MGNTL KEMQDVQGAL QCYTRAIQIN PAFADAHSNL ASIHKDSGNI PEAIASYRTA LKLKPDFPDA YCNLAHCLQI VCDWT DYDE RMKKLVSIVA DQLEKNRLPS VHPHHSMLYP LSHGFRKAIA ERHGNLCLDK INVLHKPPYE HPKDLKLSDG RLRVGY VSS DFGNHPTSHL MQSIPGMHNP DKFEVFCYAL SPDDGTNFRV KVMAEANHFI DLSQIPCNGK AADRIHQDGI HILVNMN GY TKGARNELFA LRPAPIQAMW LGYPGTSGAL FMDYIITDQE TSPAEVAEQY SEKLAYMPHT FFIGDHANMF PHLKKKAV I DFKSNGHIYD NRIVLNGIDL KAFLDSLPDV KIVKMKCPDG GDNADSSNTA LNMPVIPMNT IAEAVIEMIN RGQIQITIN GFSISNGLAT TQINNKAATG EEVPRTIIVT TRSQYGLPED AIVYCNFNQL YKIDPSTLQM WANILKRVPN SVLWLLRFPA VGEPNIQQY AQNMGLPQNR IIFSPVAPKE EHVRRGQLAD VCLDTPLCNG HTTGMDVLWA GTPMVTMPGE TLASRVAASQ L TCLGCLEL IAKNRQEYED IAVKLGTDLE YLKKVRGKVW KQRISSPLFN TKQYTMELER LYLQMWEHYA AGNKPDHMIK PV EVTESAH HHHHH UniProtKB: UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Specialist optics | Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 5539 / Average exposure time: 0.0725 sec. / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Particle selection | Number selected: 6266880 |
|---|---|
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: Details: 1W3B, 3PE3 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: cryoSPARC |
| Final 3D classification | Software - Name: cryoSPARC |
| Final angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: cryoSPARC |
| Final reconstruction | Applied symmetry - Point group: C2 (2 fold cyclic ) / Resolution.type: BY AUTHOR / Resolution: 3.82 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 124155 ) / Resolution.type: BY AUTHOR / Resolution: 3.82 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 124155 |
 Movie
Movie Controller
Controller










 Z
Z Y
Y X
X


















