+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30345 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the INT-777-bound GPBAR-Gs complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationG protein-coupled bile acid receptor activity / cell surface bile acid receptor signaling pathway / positive regulation of cholangiocyte proliferation / bile acid receptor activity / cellular response to bile acid / Class A/1 (Rhodopsin-like receptors) / regulation of bicellular tight junction assembly / PKA activation in glucagon signalling / hair follicle placode formation / developmental growth ...G protein-coupled bile acid receptor activity / cell surface bile acid receptor signaling pathway / positive regulation of cholangiocyte proliferation / bile acid receptor activity / cellular response to bile acid / Class A/1 (Rhodopsin-like receptors) / regulation of bicellular tight junction assembly / PKA activation in glucagon signalling / hair follicle placode formation / developmental growth /  D1 dopamine receptor binding / D1 dopamine receptor binding /  intracellular transport / Hedgehog 'off' state / positive regulation of cAMP-mediated signaling / adenylate cyclase-activating adrenergic receptor signaling pathway / activation of adenylate cyclase activity / adenylate cyclase activator activity / trans-Golgi network membrane / Olfactory Signaling Pathway / G-protein beta/gamma-subunit complex binding / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / intracellular transport / Hedgehog 'off' state / positive regulation of cAMP-mediated signaling / adenylate cyclase-activating adrenergic receptor signaling pathway / activation of adenylate cyclase activity / adenylate cyclase activator activity / trans-Golgi network membrane / Olfactory Signaling Pathway / G-protein beta/gamma-subunit complex binding / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor /  bone development / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / bone development / G-protein activation / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / ADP signalling through P2Y purinoceptor 12 / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Sensory perception of sweet, bitter, and umami (glutamate) taste / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion /  platelet aggregation / platelet aggregation /  cognition / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of GTPase activity / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding / Inactivation, recovery and regulation of the phototransduction cascade / cognition / Glucagon-type ligand receptors / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of GTPase activity / G alpha (z) signalling events / cellular response to catecholamine stimulus / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / cellular response to prostaglandin E stimulus / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / sensory perception of taste / GPER1 signaling / G-protein beta-subunit binding / Inactivation, recovery and regulation of the phototransduction cascade /  heterotrimeric G-protein complex / G alpha (12/13) signalling events / heterotrimeric G-protein complex / G alpha (12/13) signalling events /  extracellular vesicle / signaling receptor complex adaptor activity / sensory perception of smell / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye / extracellular vesicle / signaling receptor complex adaptor activity / sensory perception of smell / Thrombin signalling through proteinase activated receptors (PARs) / retina development in camera-type eye /  GTPase binding / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cold-induced thermogenesis / G alpha (i) signalling events / fibroblast proliferation / G alpha (s) signalling events / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / positive regulation of ERK1 and ERK2 cascade / GTPase binding / Ca2+ pathway / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cold-induced thermogenesis / G alpha (i) signalling events / fibroblast proliferation / G alpha (s) signalling events / G alpha (q) signalling events / Ras protein signal transduction / cell population proliferation / Extra-nuclear estrogen signaling / positive regulation of ERK1 and ERK2 cascade /  receptor complex / G protein-coupled receptor signaling pathway / lysosomal membrane / receptor complex / G protein-coupled receptor signaling pathway / lysosomal membrane /  GTPase activity / GTPase activity /  synapse / protein-containing complex binding / GTP binding / synapse / protein-containing complex binding / GTP binding /  signal transduction / extracellular exosome / signal transduction / extracellular exosome /  membrane / membrane /  metal ion binding / metal ion binding /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
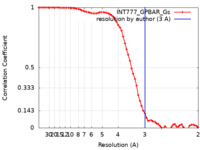
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.0 Å cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Yang F / Mao C / Guo L / Lin J / Ming Q / Xiao P / Wu X / Shen Q / Guo S / Shen D ...Yang F / Mao C / Guo L / Lin J / Ming Q / Xiao P / Wu X / Shen Q / Guo S / Shen D / Lu R / Zhang L / Huang S / Ping Y / Zhang C / Ma C / Zhang K / Liang X / Shen Y / Nan F / Yi F / Luca V / Zhou J / Jiang C / Sun J / Xie X / Yu X / Zhang Y | |||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Structural basis of GPBAR activation and bile acid recognition. Authors: Fan Yang / Chunyou Mao / Lulu Guo / Jingyu Lin / Qianqian Ming / Peng Xiao / Xiang Wu / Qingya Shen / Shimeng Guo / Dan-Dan Shen / Ruirui Lu / Linqi Zhang / Shenming Huang / Yuqi Ping / ...Authors: Fan Yang / Chunyou Mao / Lulu Guo / Jingyu Lin / Qianqian Ming / Peng Xiao / Xiang Wu / Qingya Shen / Shimeng Guo / Dan-Dan Shen / Ruirui Lu / Linqi Zhang / Shenming Huang / Yuqi Ping / Chenlu Zhang / Cheng Ma / Kai Zhang / Xiaoying Liang / Yuemao Shen / Fajun Nan / Fan Yi / Vincent C Luca / Jiuyao Zhou / Changtao Jiang / Jin-Peng Sun / Xin Xie / Xiao Yu / Yan Zhang /   Abstract: The G-protein-coupled bile acid receptor (GPBAR) conveys the cross-membrane signalling of a vast variety of bile acids and is a signalling hub in the liver-bile acid-microbiota-metabolism axis. Here ...The G-protein-coupled bile acid receptor (GPBAR) conveys the cross-membrane signalling of a vast variety of bile acids and is a signalling hub in the liver-bile acid-microbiota-metabolism axis. Here we report the cryo-electron microscopy structures of GPBAR-G complexes stabilized by either the high-affinity P395 or the semisynthesized bile acid derivative INT-777 at 3 Å resolution. These structures revealed a large oval pocket that contains several polar groups positioned to accommodate the amphipathic cholic core of bile acids, a fingerprint of key residues to recognize diverse bile acids in the orthosteric site, a putative second bile acid-binding site with allosteric properties and structural features that contribute to bias properties. Moreover, GPBAR undertakes an atypical mode of activation and G protein coupling that features a different set of key residues connecting the ligand-binding pocket to the G-coupling site, and a specific interaction motif that is localized in intracellular loop 3. Overall, our study not only reveals unique structural features of GPBAR that are involved in bile acid recognition and allosteric effects, but also suggests the presence of distinct connecting mechanisms between the ligand-binding pocket and the G-protein-binding site in the G-protein-coupled receptor superfamily. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30345.map.gz emd_30345.map.gz | 17.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30345-v30.xml emd-30345-v30.xml emd-30345.xml emd-30345.xml | 21.6 KB 21.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30345_fsc.xml emd_30345_fsc.xml | 6.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_30345.png emd_30345.png | 48.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30345 http://ftp.pdbj.org/pub/emdb/structures/EMD-30345 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30345 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30345 | HTTPS FTP |
-Related structure data
| Related structure data |  7cfnMC  7cfmC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30345.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30345.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : INT-777-bound GPBAR-Gs complex
+Supramolecule #1: INT-777-bound GPBAR-Gs complex
+Supramolecule #2: Guanine nucleotide-binding protein subunits
+Supramolecule #3: Nanobody-35
+Supramolecule #4: G-protein coupled bile acid receptor 1
+Macromolecule #1: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
+Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
+Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
+Macromolecule #4: Nanobody-35
+Macromolecule #5: G-protein coupled bile acid receptor 1
+Macromolecule #6: (2S,4R)-4-[(3R,5S,6R,7R,8R,9S,10S,12S,13R,14S,17R)-6-ethyl-10,13-...
+Macromolecule #7: CHOLESTEROL
+Macromolecule #8: PALMITIC ACID
+Macromolecule #9: water
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 49310 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal magnification: 29000 Bright-field microscopy / Cs: 2.7 mm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number real images: 6229 / Average electron dose: 62.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller