[English] 日本語
 Yorodumi
Yorodumi- EMDB-27659: cryoEM map of de novo hallucinated homooligomer of C18-C6 symmetr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryoEM map of de novo hallucinated homooligomer of C18-C6 symmetry, design HALC18-6_265. | ||||||||||||
 Map data Map data | EM map | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords |  De novo protein design / De novo protein design /  Computational protein design / Computational protein design /  Machine learning / Hallucination / homo-oligomers / Machine learning / Hallucination / homo-oligomers /  DE NOVO PROTEIN DE NOVO PROTEIN | ||||||||||||
| Biological species |   Escherichia coli (E. coli) / Escherichia coli (E. coli) /   Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) | ||||||||||||
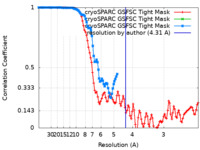
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.31 Å cryo EM / Resolution: 4.31 Å | ||||||||||||
 Authors Authors | Wicky BIM / Milles LF / Courbet AC / Baker D | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Science / Year: 2022 Journal: Science / Year: 2022Title: Hallucinating symmetric protein assemblies. Authors: B I M Wicky / L F Milles / A Courbet / R J Ragotte / J Dauparas / E Kinfu / S Tipps / R D Kibler / M Baek / F DiMaio / X Li / L Carter / A Kang / H Nguyen / A K Bera / D Baker /  Abstract: Deep learning generative approaches provide an opportunity to broadly explore protein structure space beyond the sequences and structures of natural proteins. Here, we use deep network hallucination ...Deep learning generative approaches provide an opportunity to broadly explore protein structure space beyond the sequences and structures of natural proteins. Here, we use deep network hallucination to generate a wide range of symmetric protein homo-oligomers given only a specification of the number of protomers and the protomer length. Crystal structures of seven designs are very similar to the computational models (median root mean square deviation: 0.6 angstroms), as are three cryo-electron microscopy structures of giant 10-nanometer rings with up to 1550 residues and symmetry; all differ considerably from previously solved structures. Our results highlight the rich diversity of new protein structures that can be generated using deep learning and pave the way for the design of increasingly complex components for nanomachines and biomaterials. #1:  Journal: BioRxiv / Year: 2022 Journal: BioRxiv / Year: 2022Title: Hallucinating protein assemblies Authors: Wicky BIM / Milles LF / Courbet A / Baker D | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27659.map.gz emd_27659.map.gz | 117.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27659-v30.xml emd-27659-v30.xml emd-27659.xml emd-27659.xml | 16.1 KB 16.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27659_fsc.xml emd_27659_fsc.xml emd_27659_fsc_2.xml emd_27659_fsc_2.xml emd_27659_fsc_3.xml emd_27659_fsc_3.xml | 10.5 KB 5.9 KB 5.9 KB | Display Display Display |  FSC data file FSC data file |
| Images |  emd_27659.png emd_27659.png | 54.4 KB | ||
| Masks |  emd_27659_msk_1.map emd_27659_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-27659.cif.gz emd-27659.cif.gz | 4.8 KB | ||
| Others |  emd_27659_half_map_1.map.gz emd_27659_half_map_1.map.gz emd_27659_half_map_2.map.gz emd_27659_half_map_2.map.gz | 116.1 MB 116.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27659 http://ftp.pdbj.org/pub/emdb/structures/EMD-27659 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27659 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27659 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_27659.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27659.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27659_msk_1.map emd_27659_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
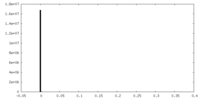
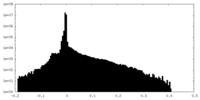
| Density Histograms |
-Half map: Half map A
| File | emd_27659_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
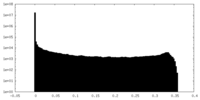
| Density Histograms |
-Half map: Half map B
| File | emd_27659_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
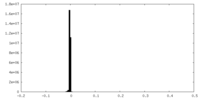
| Density Histograms |
- Sample components
Sample components
-Entire : De novo hallucinated homooligomer of C18-C6 symmetry, design HALC...
| Entire | Name: De novo hallucinated homooligomer of C18-C6 symmetry, design HALC18-6_265. |
|---|---|
| Components |
|
-Supramolecule #1: De novo hallucinated homooligomer of C18-C6 symmetry, design HALC...
| Supramolecule | Name: De novo hallucinated homooligomer of C18-C6 symmetry, design HALC18-6_265. type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Macromolecule #1: HALC18-6_265
| Macromolecule | Name: HALC18-6_265 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) |
| Recombinant expression | Organism:   Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) Escherichia coli 'BL21-Gold(DE3)pLysS AG' (bacteria) |
| Sequence | String: NIKIPNPKDL SELLKKLGEG LKGLPNLKTL TLTLSNIELP EDADLSPGAE GLGEGLKGLP NLETLTFTIS NIKIPNPKD LSELLKKLGE GLKGLPNLKT LTLTLSNIEL PEDADLSPGA EGLGEGLKGL PNLETLTFTI S NIKIPNPK DLSELLKKLG EGLKGLPNLK ...String: NIKIPNPKDL SELLKKLGEG LKGLPNLKTL TLTLSNIELP EDADLSPGAE GLGEGLKGLP NLETLTFTIS NIKIPNPKD LSELLKKLGE GLKGLPNLKT LTLTLSNIEL PEDADLSPGA EGLGEGLKGL PNLETLTFTI S NIKIPNPK DLSELLKKLG EGLKGLPNLK TLTLTLSNIE LPEDADLSPG AEGLGEGLKG LPNLETLTFT IS |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8.4 Component:
| |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X