+ Open data
Open data
- Basic information
Basic information
| Entry | 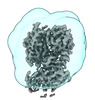 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cereblon bound to CC-92480, Focused Refinement | |||||||||
 Map data Map data | focused refinement of CRBN~DDB1 with Mezigdomide | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.1 Å cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Watson ER / Lander GC | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2022 Journal: Science / Year: 2022Title: Molecular glue CELMoD compounds are regulators of cereblon conformation. Authors: Edmond R Watson / Scott Novick / Mary E Matyskiela / Philip P Chamberlain / Andres H de la Peña / Jinyi Zhu / Eileen Tran / Patrick R Griffin / Ingrid E Wertz / Gabriel C Lander /  Abstract: Cereblon (CRBN) is a ubiquitin ligase (E3) substrate receptor protein co-opted by CRBN E3 ligase modulatory drug (CELMoD) agents that target therapeutically relevant proteins for degradation. Prior ...Cereblon (CRBN) is a ubiquitin ligase (E3) substrate receptor protein co-opted by CRBN E3 ligase modulatory drug (CELMoD) agents that target therapeutically relevant proteins for degradation. Prior crystallographic studies defined the drug-binding site within CRBN's thalidomide-binding domain (TBD), but the allostery of drug-induced neosubstrate binding remains unclear. We performed cryo-electron microscopy analyses of the DNA damage-binding protein 1 (DDB1)-CRBN apo complex and compared these structures with DDB1-CRBN in the presence of CELMoD compounds alone and complexed with neosubstrates. Association of CELMoD compounds to the TBD is necessary and sufficient for triggering CRBN allosteric rearrangement from an open conformation to the canonical closed conformation. The neosubstrate Ikaros only stably associates with the closed CRBN conformation, illustrating the importance of allostery for CELMoD compound efficacy and informing structure-guided design strategies to improve therapeutic efficacy. #1:  Journal: Biorxiv / Year: 2022 Journal: Biorxiv / Year: 2022Title: Molecular glue CELMoD compounds are allosteric regulators of cereblon conformation Authors: Watson ER / Novick SJ / Matyskiela ME / Chamberlain PP / de la Pena AH / Zhu JY / Tran E / Griffin PR / Wertz IE / Lander GC | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27239.map.gz emd_27239.map.gz | 40.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27239-v30.xml emd-27239-v30.xml emd-27239.xml emd-27239.xml | 21.1 KB 21.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27239_fsc.xml emd_27239_fsc.xml | 7.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_27239.png emd_27239.png | 112.4 KB | ||
| Masks |  emd_27239_msk_1.map emd_27239_msk_1.map | 42.9 MB |  Mask map Mask map | |
| Others |  emd_27239_additional_1.map.gz emd_27239_additional_1.map.gz emd_27239_additional_2.map.gz emd_27239_additional_2.map.gz emd_27239_half_map_1.map.gz emd_27239_half_map_1.map.gz emd_27239_half_map_2.map.gz emd_27239_half_map_2.map.gz | 21.7 MB 38 MB 39.8 MB 39.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27239 http://ftp.pdbj.org/pub/emdb/structures/EMD-27239 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27239 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27239 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_27239.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27239.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | focused refinement of CRBN~DDB1 with Mezigdomide | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.15 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27239_msk_1.map emd_27239_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
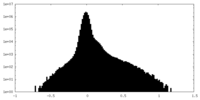
| Density Histograms |
-Additional map: #1
| File | emd_27239_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Postprocessed with DeepEMhancer
| File | emd_27239_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed with DeepEMhancer | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_27239_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_27239_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cereblon bound to CC-92480
| Entire | Name: Cereblon bound to CC-92480 |
|---|---|
| Components |
|
-Supramolecule #1: Cereblon bound to CC-92480
| Supramolecule | Name: Cereblon bound to CC-92480 / type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Cereblon
| Macromolecule | Name: Cereblon / type: protein_or_peptide / ID: 1 / Details: bound to CC-92480 mezigdomide ligand / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MAGEGDQQDA AHNMGNHLPL LPAESEEEDE MEVEDQDSKE AKKPNIINFD TSLPTSHTYL GADMEEFHGR TLHDDDSCQV IPVLPQVMMI LIPGQTLPLQ LFHPQEVSMV RNLIQKDRTF AVLAYSNVQE REAQFGTTAE IYAYREEQDF GIEIVKVKAI GRQRFKVLEL ...String: MAGEGDQQDA AHNMGNHLPL LPAESEEEDE MEVEDQDSKE AKKPNIINFD TSLPTSHTYL GADMEEFHGR TLHDDDSCQV IPVLPQVMMI LIPGQTLPLQ LFHPQEVSMV RNLIQKDRTF AVLAYSNVQE REAQFGTTAE IYAYREEQDF GIEIVKVKAI GRQRFKVLEL RTQSDGIQQA KVQILPECVL PSTMSAVQLE SLNKCQIFPS KPVSREDQCS YKWWQKYQKR KFHCANLTSW PRWLYSLYDA ETLMDRIKKQ LREWDENLKD DSLPSNPIDF SYRVAACLPI DDVLRIQLLK IGSAIQRLRC ELDIMNKCTS LCCKQCQETE ITTKNEIFSL SLCGPMAAYV NPHGYVHETL TVYKACNLNL IGRPSTEHSW FPGYAWTVAQ CKICASHIGW KFTATKKDMS PQKFWGLTRS ALLPTIPDTE DEISPDKVIL CL |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 20 mg/mL |
|---|---|
| Buffer | pH: 7 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 25 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.8 µm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 62.5 e/Å2 |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z
Z Y
Y X
X