[English] 日本語
 Yorodumi
Yorodumi- EMDB-25377: Cryo-EM structure of mouse agonist ML-SA1-bound TRPML1 channel at... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-25377 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of mouse agonist ML-SA1-bound TRPML1 channel at 2.32 Angstrom resolution | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationTransferrin endocytosis and recycling / positive regulation of lysosome organization / calcium ion export / intracellularly phosphatidylinositol-3,5-bisphosphate-gated monatomic cation channel activity / phagosome maturation / NAADP-sensitive calcium-release channel activity / cellular response to pH / ligand-gated calcium channel activity /  TRP channels / endosomal transport ...Transferrin endocytosis and recycling / positive regulation of lysosome organization / calcium ion export / intracellularly phosphatidylinositol-3,5-bisphosphate-gated monatomic cation channel activity / phagosome maturation / NAADP-sensitive calcium-release channel activity / cellular response to pH / ligand-gated calcium channel activity / TRP channels / endosomal transport ...Transferrin endocytosis and recycling / positive regulation of lysosome organization / calcium ion export / intracellularly phosphatidylinositol-3,5-bisphosphate-gated monatomic cation channel activity / phagosome maturation / NAADP-sensitive calcium-release channel activity / cellular response to pH / ligand-gated calcium channel activity /  TRP channels / endosomal transport / phagocytic cup / intracellular vesicle / autophagosome maturation / monoatomic cation transmembrane transport / localization / release of sequestered calcium ion into cytosol / monoatomic cation channel activity / cellular response to calcium ion / cell projection / calcium ion transmembrane transport / phagocytic vesicle membrane / late endosome / late endosome membrane / protein homotetramerization / TRP channels / endosomal transport / phagocytic cup / intracellular vesicle / autophagosome maturation / monoatomic cation transmembrane transport / localization / release of sequestered calcium ion into cytosol / monoatomic cation channel activity / cellular response to calcium ion / cell projection / calcium ion transmembrane transport / phagocytic vesicle membrane / late endosome / late endosome membrane / protein homotetramerization /  adaptive immune response / adaptive immune response /  lysosome / lysosome /  receptor complex / lysosomal membrane / intracellular membrane-bounded organelle / receptor complex / lysosomal membrane / intracellular membrane-bounded organelle /  lipid binding / lipid binding /  Golgi apparatus / Golgi apparatus /  nucleoplasm / nucleoplasm /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.32 Å cryo EM / Resolution: 2.32 Å | |||||||||
 Authors Authors | Gan N / Han Y / Jiang Y | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: Structural mechanism of allosteric activation of TRPML1 by PI(3,5)P and rapamycin. Authors: Ninghai Gan / Yan Han / Weizhong Zeng / Yan Wang / Jing Xue / Youxing Jiang /  Abstract: Transient receptor potential mucolipin 1 (TRPML1) is a Ca-permeable, nonselective cation channel ubiquitously expressed in the endolysosomes of mammalian cells and its loss-of-function mutations are ...Transient receptor potential mucolipin 1 (TRPML1) is a Ca-permeable, nonselective cation channel ubiquitously expressed in the endolysosomes of mammalian cells and its loss-of-function mutations are the direct cause of type IV mucolipidosis (MLIV), an autosomal recessive lysosomal storage disease. TRPML1 is a ligand-gated channel that can be activated by phosphatidylinositol 3,5-bisphosphate [PI(3,5)P] as well as some synthetic small-molecule agonists. Recently, rapamycin has also been shown to directly bind and activate TRPML1. Interestingly, both PI(3,5)P and rapamycin have low efficacy in channel activation individually but together they work cooperatively and activate the channel with high potency. To reveal the structural basis underlying the synergistic activation of TRPML1 by PI(3,5)P and rapamycin, we determined the high-resolution cryoelectron microscopy (cryo-EM) structures of the mouse TRPML1 channel in various states, including apo closed, PI(3,5)P-bound closed, and PI(3,5)P/temsirolimus (a rapamycin analog)-bound open states. These structures, combined with electrophysiology, elucidate the molecular details of ligand binding and provide structural insight into how the TRPML1 channel integrates two distantly bound ligand stimuli and facilitates channel opening. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25377.map.gz emd_25377.map.gz | 84.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25377-v30.xml emd-25377-v30.xml emd-25377.xml emd-25377.xml | 11 KB 11 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25377.png emd_25377.png | 50 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25377 http://ftp.pdbj.org/pub/emdb/structures/EMD-25377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25377 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25377 | HTTPS FTP |
-Related structure data
| Related structure data |  7sq6MC  7sq7C  7sq8C  7sq9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_25377.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25377.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Mouse Transient receptor potential-mucolipin 1 apo closed state
| Entire | Name: Mouse Transient receptor potential-mucolipin 1 apo closed state |
|---|---|
| Components |
|
-Supramolecule #1: Mouse Transient receptor potential-mucolipin 1 apo closed state
| Supramolecule | Name: Mouse Transient receptor potential-mucolipin 1 apo closed state type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Mucolipin-1
| Macromolecule | Name: Mucolipin-1 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Molecular weight | Theoretical: 65.573617 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MATPAGRRAS ETERLLTPNP GYGTQVGTSP APTTPTEEED LRRRLKYFFM SPCDKFRAKG RKPCKLMLQV VKILVVTVQL ILFGLSNQL VVTFREENTI AFRHLFLLGY SDGSDDTFAA YTQEQLYQAI FYAVDQYLIL PEISLGRYAY VRGGGGPWAN G SALALCQR ...String: MATPAGRRAS ETERLLTPNP GYGTQVGTSP APTTPTEEED LRRRLKYFFM SPCDKFRAKG RKPCKLMLQV VKILVVTVQL ILFGLSNQL VVTFREENTI AFRHLFLLGY SDGSDDTFAA YTQEQLYQAI FYAVDQYLIL PEISLGRYAY VRGGGGPWAN G SALALCQR YYHRGHVDPA NDTFDIDPRV VTDCIQVDPP DRPPDIPSED LDFLDGSASY KNLTLKFHKL INVTIHFQLK TI NLQSLIN NEIPDCYTFS ILITFDNKAH SGRIPIRLET KTHIQECKHP SVSRHGDNSF RLLFDVVVIL TCSLSFLLCA RSL LRGFLL QNEFVVFMWR RRGREISLWE RLEFVNGWYI LLVTSDVLTI SGTVMKIGIE AKNLASYDVC SILLGTSTLL VWVG VIRYL TFFHKYNILI ATLRVALPSV MRFCCCVAVI YLGYCFCGWI VLGPYHVKFR SLSMVSECLF SLINGDDMFV TFAAM QAQQ GHSSLVWLFS QLYLYSFISL FIYMVLSLFI ALITGAYDTI KHPGGTGTEK SELQAYIEQC QDSPTSGKFR RGSGSA CSL FCCCGRDSPE DHSLLVN |
-Macromolecule #3: 2-{2-oxo-2-[(4S)-2,2,4-trimethyl-3,4-dihydroquinolin-1(2H)-yl]eth...
| Macromolecule | Name: 2-{2-oxo-2-[(4S)-2,2,4-trimethyl-3,4-dihydroquinolin-1(2H)-yl]ethyl}-1H-isoindole-1,3(2H)-dione type: ligand / ID: 3 / Number of copies: 4 / Formula: AQV |
|---|---|
| Molecular weight | Theoretical: 362.422 Da |
| Chemical component information | 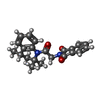 ChemComp-AQV: |
-Macromolecule #4: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 4 / Number of copies: 2 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 12 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.9 µm Bright-field microscopy / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.9 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
|---|---|
| Final angle assignment | Type: RANDOM ASSIGNMENT |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.32 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 41980 |
 Movie
Movie Controller
Controller