[English] 日本語
 Yorodumi
Yorodumi- EMDB-2364: CryoEM reconstruction of the bacteriophage phi6 procapsid to the ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2364 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM reconstruction of the bacteriophage phi6 procapsid to the near-atomic resolution | |||||||||
 Map data Map data | Reconstruction of the wildtype P1247 procapsid of bacteriophage phi6 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Bacteriophage phi6 /  Cystoviridae / capsid structure / capsid expansion / segmented genome / Cystoviridae / capsid structure / capsid expansion / segmented genome /  conformational change / RNA packaging conformational change / RNA packaging | |||||||||
| Function / homology | : / Major inner capsid protein P1 / T=2 icosahedral viral capsid / viral inner capsid / viral nucleocapsid /  RNA binding / identical protein binding / Major inner protein P1 RNA binding / identical protein binding / Major inner protein P1 Function and homology information Function and homology information | |||||||||
| Biological species |   Pseudomonas phage phi6 (bacteriophage) Pseudomonas phage phi6 (bacteriophage) | |||||||||
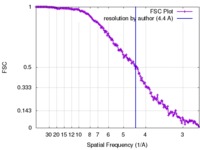
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.4 Å cryo EM / Resolution: 4.4 Å | |||||||||
 Authors Authors | Nemecek D / Boura E / Wu W / Cheng N / Plevka P / Qiao J / Mindich L / Heymann JB / Hurley JH / Steven AC | |||||||||
 Citation Citation |  Journal: Structure / Year: 2013 Journal: Structure / Year: 2013Title: Subunit folds and maturation pathway of a dsRNA virus capsid. Authors: Daniel Nemecek / Evzen Boura / Weimin Wu / Naiqian Cheng / Pavel Plevka / Jian Qiao / Leonard Mindich / J Bernard Heymann / James H Hurley / Alasdair C Steven /  Abstract: The cystovirus ϕ6 shares several distinct features with other double-stranded RNA (dsRNA) viruses, including the human pathogen, rotavirus: segmented genomes, nonequivalent packing of 120 subunits ...The cystovirus ϕ6 shares several distinct features with other double-stranded RNA (dsRNA) viruses, including the human pathogen, rotavirus: segmented genomes, nonequivalent packing of 120 subunits in its icosahedral capsid, and capsids as compartments for transcription and replication. ϕ6 assembles as a dodecahedral procapsid that undergoes major conformational changes as it matures into the spherical capsid. We determined the crystal structure of the capsid protein, P1, revealing a flattened trapezoid subunit with an α-helical fold. We also solved the procapsid with cryo-electron microscopy to comparable resolution. Fitting the crystal structure into the procapsid disclosed substantial conformational differences between the two P1 conformers. Maturation via two intermediate states involves remodeling on a similar scale, besides huge rigid-body rotations. The capsid structure and its stepwise maturation that is coupled to sequential packaging of three RNA segments sets the cystoviruses apart from other dsRNA viruses as a dynamic molecular machine. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2364.map.gz emd_2364.map.gz | 265.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2364-v30.xml emd-2364-v30.xml emd-2364.xml emd-2364.xml | 15.1 KB 15.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_2364_fsc.xml emd_2364_fsc.xml | 15.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_2364.jpg emd_2364.jpg | 193.8 KB | ||
| Masks |  emd_2364_msk_1.map emd_2364_msk_1.map emd_2364_msk_2.map emd_2364_msk_2.map | 70.7 MB 70.7 MB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2364 http://ftp.pdbj.org/pub/emdb/structures/EMD-2364 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2364 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2364 | HTTPS FTP |
-Related structure data
| Related structure data |  4btgMC  4btqC  4k7hC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_2364.map.gz / Format: CCP4 / Size: 276 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2364.map.gz / Format: CCP4 / Size: 276 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of the wildtype P1247 procapsid of bacteriophage phi6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.397 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Segmentation: This mask represents the P1B subunit
| Annotation | This mask represents the P1B subunit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_2364_msk_1.map emd_2364_msk_1.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Segmentation: This mask represents the P1A subunit
| Annotation | This mask represents the P1A subunit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_2364_msk_2.map emd_2364_msk_2.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Wildtype P1247 procapsid of bacteriophage phi6
| Entire | Name: Wildtype P1247 procapsid of bacteriophage phi6 |
|---|---|
| Components |
|
-Supramolecule #1000: Wildtype P1247 procapsid of bacteriophage phi6
| Supramolecule | Name: Wildtype P1247 procapsid of bacteriophage phi6 / type: sample / ID: 1000 / Oligomeric state: icosahedral shell with accessory proteins / Number unique components: 4 |
|---|---|
| Molecular weight | Theoretical: 12.6 MDa |
-Supramolecule #1: Pseudomonas phage phi6
| Supramolecule | Name: Pseudomonas phage phi6 / type: virus / ID: 1 / Name.synonym: bacteriophage phi-6 / NCBI-ID: 10879 / Sci species name: Pseudomonas phage phi6 / Virus type: VIRION / Virus isolate: SPECIES / Virus enveloped: No / Virus empty: Yes / Syn species name: bacteriophage phi-6 |
|---|---|
| Host (natural) | Organism:   Pseudomonas syringae (bacteria) / synonym: BACTERIA(EUBACTERIA) Pseudomonas syringae (bacteria) / synonym: BACTERIA(EUBACTERIA) |
| Host system | Organism:   Escherichia coli (E. coli) / Recombinant strain: JM109 / Recombinant plasmid: pLM687 Escherichia coli (E. coli) / Recombinant strain: JM109 / Recombinant plasmid: pLM687 |
| Molecular weight | Theoretical: 12.6 MDa |
| Virus shell | Shell ID: 1 / Name: P1247 / Diameter: 450 Å / T number (triangulation number): 2 |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 10 mM potassium phosphate, 5 mM MgCl2 |
| Grid | Details: 400 mesh C-flat holey carbon grid |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 100 K / Instrument: FEI VITROBOT MARK I / Method: Blot for 2 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 44739 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 46000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 46000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Date | Nov 20, 2011 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: NIKON SUPER COOLSCAN 9000 / Digitization - Sampling interval: 6.35 µm / Number real images: 220 / Average electron dose: 15 e/Å2 / Bits/pixel: 16 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Software | Name: Chimera, MDFF, Coot |
| Details | the P1 crystal structure was rigid body-fitted into the procapsid map using Chimera. Regions where the crystal structure deviated significantly from the EM density were roughly adjusted in Coot and then the P1A and P1B structures were flexibly fitted using the MDFF package. Finally, both structures were visually inspected and refined in Coot. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-4btg: |
 Movie
Movie Controller
Controller










 Z
Z Y
Y X
X