[English] 日本語
 Yorodumi
Yorodumi- EMDB-1893: EcoKI Type I DNA restriction-modification enzyme complex in close... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1893 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
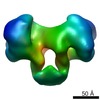
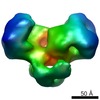
| Title | EcoKI Type I DNA restriction-modification enzyme complex in closed state with bound 75bp cognate DNA fragment. 3D reconstruction by single particle analysis from negative stain EM. | |||||||||
 Map data Map data | 3D reconstruction of EcoKI Type I restriction-modification enzyme with DNA fragment, from negative stain EM data. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | EcoKI /  endonuclease / endonuclease /  methyltransferase / type I restriction / methyltransferase / type I restriction /  DNA / DNA /  HsdS / HsdM / HsdR / HsdS / HsdM / HsdR /  electron microscopy / electron microscopy /  negative stain / negative stain /  translocase / DEAD-box / translocase / DEAD-box /  ATPase ATPase | |||||||||
| Function / homology | Type I restriction modification DNA specificity domain /  Restriction endonuclease, type I, HsdR / Restriction endonuclease, type I, HsdR /  protein binding / DNA methylase, adenine-specific / DNA restriction-modification system protein binding / DNA methylase, adenine-specific / DNA restriction-modification system Function and homology information Function and homology information | |||||||||
| Biological species |   Escherichia coli (E. coli) / synthetic construct (others) Escherichia coli (E. coli) / synthetic construct (others) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  negative staining / Resolution: 20.0 Å negative staining / Resolution: 20.0 Å | |||||||||
 Authors Authors | Kennaway CK / Taylor JE / Song CF / Potrzebowski W / White JH / Swiderska A / Obarska-Kosinska A / Callow P / Cooper LP / Roberts GA ...Kennaway CK / Taylor JE / Song CF / Potrzebowski W / White JH / Swiderska A / Obarska-Kosinska A / Callow P / Cooper LP / Roberts GA / Bujnicki JM / Trinick J / Kneale GG / Dryden DTF | |||||||||
 Citation Citation |  Journal: Genes Dev / Year: 2012 Journal: Genes Dev / Year: 2012Title: Structure and operation of the DNA-translocating type I DNA restriction enzymes. Authors: Christopher K Kennaway / James E N Taylor / Chun Feng Song / Wojciech Potrzebowski / William Nicholson / John H White / Anna Swiderska / Agnieszka Obarska-Kosinska / Philip Callow / Laurie P ...Authors: Christopher K Kennaway / James E N Taylor / Chun Feng Song / Wojciech Potrzebowski / William Nicholson / John H White / Anna Swiderska / Agnieszka Obarska-Kosinska / Philip Callow / Laurie P Cooper / Gareth A Roberts / Jean-Baptiste Artero / Janusz M Bujnicki / John Trinick / G Geoff Kneale / David T F Dryden /    Abstract: Type I DNA restriction/modification (RM) enzymes are molecular machines found in the majority of bacterial species. Their early discovery paved the way for the development of genetic engineering. ...Type I DNA restriction/modification (RM) enzymes are molecular machines found in the majority of bacterial species. Their early discovery paved the way for the development of genetic engineering. They control (restrict) the influx of foreign DNA via horizontal gene transfer into the bacterium while maintaining sequence-specific methylation (modification) of host DNA. The endonuclease reaction of these enzymes on unmethylated DNA is preceded by bidirectional translocation of thousands of base pairs of DNA toward the enzyme. We present the structures of two type I RM enzymes, EcoKI and EcoR124I, derived using electron microscopy (EM), small-angle scattering (neutron and X-ray), and detailed molecular modeling. DNA binding triggers a large contraction of the open form of the enzyme to a compact form. The path followed by DNA through the complexes is revealed by using a DNA mimic anti-restriction protein. The structures reveal an evolutionary link between type I RM enzymes and type II RM enzymes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1893.map.gz emd_1893.map.gz | 80.6 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1893-v30.xml emd-1893-v30.xml emd-1893.xml emd-1893.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
| Images |  1893.png 1893.png | 154.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1893 http://ftp.pdbj.org/pub/emdb/structures/EMD-1893 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1893 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1893 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1893.map.gz / Format: CCP4 / Size: 422.9 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1893.map.gz / Format: CCP4 / Size: 422.9 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D reconstruction of EcoKI Type I restriction-modification enzyme with DNA fragment, from negative stain EM data. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 5.27 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : EcoKI R2 M2 S1 complex with 75 bp cognate dsDNA fragment
| Entire | Name: EcoKI R2 M2 S1 complex with 75 bp cognate dsDNA fragment |
|---|---|
| Components |
|
-Supramolecule #1000: EcoKI R2 M2 S1 complex with 75 bp cognate dsDNA fragment
| Supramolecule | Name: EcoKI R2 M2 S1 complex with 75 bp cognate dsDNA fragment type: sample / ID: 1000 / Details: Stained with uranyl acetate / Oligomeric state: 1x HsdS, 2x HsdM, 2x HsdR, 1x dsDNA / Number unique components: 4 |
|---|---|
| Molecular weight | Experimental: 420 KDa / Theoretical: 440 KDa / Method: Small angle X-ray scattering (SAXS) |
-Macromolecule #1: EcoKI HsdS specificity subunit
| Macromolecule | Name: EcoKI HsdS specificity subunit / type: protein_or_peptide / ID: 1 / Name.synonym: HsdS / Number of copies: 1 / Oligomeric state: Monomer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) / Strain: R / Location in cell: Cytoplasm Escherichia coli (E. coli) / Strain: R / Location in cell: Cytoplasm |
| Molecular weight | Theoretical: 50 KDa |
| Sequence | GO:  protein binding protein bindingInterPro: Type I restriction modification DNA specificity domain |
-Macromolecule #2: EcoKI HsdM methyltransferase subunit
| Macromolecule | Name: EcoKI HsdM methyltransferase subunit / type: protein_or_peptide / ID: 2 / Name.synonym: HsdM / Number of copies: 2 / Oligomeric state: Dimer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) / Strain: R / Location in cell: cytoplasm Escherichia coli (E. coli) / Strain: R / Location in cell: cytoplasm |
| Molecular weight | Theoretical: 59 KDa |
| Sequence | GO:  protein binding / InterPro: DNA methylase, adenine-specific protein binding / InterPro: DNA methylase, adenine-specific |
-Macromolecule #3: EcoKI HsdR endonuclease subunit
| Macromolecule | Name: EcoKI HsdR endonuclease subunit / type: protein_or_peptide / ID: 3 / Name.synonym: HsdR / Number of copies: 2 / Oligomeric state: Monomer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:   Escherichia coli (E. coli) / Strain: R Escherichia coli (E. coli) / Strain: R |
| Molecular weight | Theoretical: 120 KDa |
| Sequence | GO: DNA restriction-modification system / InterPro:  Restriction endonuclease, type I, HsdR Restriction endonuclease, type I, HsdR |
-Macromolecule #4: Deoxyribonucleic acid
| Macromolecule | Name: Deoxyribonucleic acid / type: dna / ID: 4 / Name.synonym: DNA / Classification: DNA / Structure: DOUBLE HELIX / Synthetic?: Yes |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 15 KDa |
| Sequence | String: AACNNNNNNG TGC |
-Experimental details
-Structure determination
| Method |  negative staining negative staining |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.050 mg/mL |
|---|---|
| Buffer | pH: 4.7 / Details: 20mM Tris-Cl, 100 mM NaCl |
| Staining | Type: NEGATIVE Details: Protein was adsorbed onto UV treated carbon for 1 minute, blotted, then 1% uranyl acetate solution was applied for 1 min then blotted, three times. |
| Grid | Details: 400 mesh copper with continuous carbon |
| Vitrification | Cryogen name: NONE / Instrument: OTHER / Details: Negative stain |
- Electron microscopy
Electron microscopy
| Microscope | JEOL 1200EXII |
|---|---|
| Electron beam | Acceleration voltage: 80 kV / Electron source: TUNGSTEN HAIRPIN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 0.9 µm / Nominal defocus min: 0.4 µm / Nominal magnification: 40000 Bright-field microscopy / Cs: 2.0 mm / Nominal defocus max: 0.9 µm / Nominal defocus min: 0.4 µm / Nominal magnification: 40000 |
| Sample stage | Specimen holder: Side entry / Specimen holder model: JEOL |
| Temperature | Average: 294 K |
| Alignment procedure | Legacy - Astigmatism: Corrected at 80,000x |
| Details | Customised JEOL 1200 EX microscope, low dose mode. |
| Date | Jul 1, 2004 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 20 µm / Number real images: 50 / Average electron dose: 40 e/Å2 / Details: Scanned on Imacon scanner / Bits/pixel: 8 |
- Image processing
Image processing
| CTF correction | Details: Filtered at 1st zero |
|---|---|
| Final two d classification | Number classes: 100 |
| Final angle assignment | Details: full range, C2 symmetry |
| Final reconstruction | Applied symmetry - Point group: C2 (2 fold cyclic ) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 20.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: EMAN, IMAGIC, Spider, XMIPP ) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 20.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: EMAN, IMAGIC, Spider, XMIPPDetails: Final rounds of refinement done in EMAN using selected input classes. Number images used: 5786 |
| Details | The particles were manually selected using boxer. |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A |
|---|---|
| Software | Name:  Chimera Chimera |
| Details | PDBEntryID_givenInChain. Protocol: rigid body. Homology model based on HsdR from EcoR124 (2W00) was fitted into the density after the core methylase (HsdS and 2x HsdM) was fitted. |
| Refinement | Space: RECIPROCAL / Protocol: RIGID BODY FIT / Target criteria: cross-correlation |
 Movie
Movie Controller
Controller