[English] 日本語
 Yorodumi
Yorodumi- EMDB-18374: cryo-EM structure complex of Frizzled-7 and Clostridioides diffic... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryo-EM structure complex of Frizzled-7 and Clostridioides difficile toxin B | ||||||||||||||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||||||||||||||
 Keywords Keywords | micriobiology / class F G protein-coupled receptors / CROP dynamics /  TOXIN TOXIN | ||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of ectodermal cell fate specification / negative regulation of cardiac muscle cell differentiation / mesenchymal to epithelial transition / skeletal muscle satellite cell maintenance involved in skeletal muscle regeneration /  Wnt receptor activity / somatic stem cell division / non-canonical Wnt signaling pathway / Wnt receptor activity / somatic stem cell division / non-canonical Wnt signaling pathway /  glucosyltransferase activity / Wnt-protein binding / positive regulation of epithelial cell proliferation involved in wound healing ...negative regulation of ectodermal cell fate specification / negative regulation of cardiac muscle cell differentiation / mesenchymal to epithelial transition / skeletal muscle satellite cell maintenance involved in skeletal muscle regeneration / glucosyltransferase activity / Wnt-protein binding / positive regulation of epithelial cell proliferation involved in wound healing ...negative regulation of ectodermal cell fate specification / negative regulation of cardiac muscle cell differentiation / mesenchymal to epithelial transition / skeletal muscle satellite cell maintenance involved in skeletal muscle regeneration /  Wnt receptor activity / somatic stem cell division / non-canonical Wnt signaling pathway / Wnt receptor activity / somatic stem cell division / non-canonical Wnt signaling pathway /  glucosyltransferase activity / Wnt-protein binding / positive regulation of epithelial cell proliferation involved in wound healing / WNT5:FZD7-mediated leishmania damping / glucosyltransferase activity / Wnt-protein binding / positive regulation of epithelial cell proliferation involved in wound healing / WNT5:FZD7-mediated leishmania damping /  frizzled binding / PCP/CE pathway / regulation of canonical Wnt signaling pathway / Class B/2 (Secretin family receptors) / negative regulation of cell-substrate adhesion / frizzled binding / PCP/CE pathway / regulation of canonical Wnt signaling pathway / Class B/2 (Secretin family receptors) / negative regulation of cell-substrate adhesion /  Wnt signaling pathway, planar cell polarity pathway / host cell cytosol / Wnt signaling pathway, planar cell polarity pathway / host cell cytosol /  Transferases; Glycosyltransferases; Hexosyltransferases / stem cell population maintenance / canonical Wnt signaling pathway / cysteine-type peptidase activity / cellular response to retinoic acid / positive regulation of phosphorylation / Transferases; Glycosyltransferases; Hexosyltransferases / stem cell population maintenance / canonical Wnt signaling pathway / cysteine-type peptidase activity / cellular response to retinoic acid / positive regulation of phosphorylation /  phosphatidylinositol-4,5-bisphosphate binding / substrate adhesion-dependent cell spreading / host cell endosome membrane / Asymmetric localization of PCP proteins / phosphatidylinositol-4,5-bisphosphate binding / substrate adhesion-dependent cell spreading / host cell endosome membrane / Asymmetric localization of PCP proteins /  PDZ domain binding / G protein-coupled receptor activity / positive regulation of JNK cascade / neuron differentiation / recycling endosome membrane / T cell differentiation in thymus / PDZ domain binding / G protein-coupled receptor activity / positive regulation of JNK cascade / neuron differentiation / recycling endosome membrane / T cell differentiation in thymus /  toxin activity / toxin activity /  Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / positive regulation of MAPK cascade / intracellular membrane-bounded organelle / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / positive regulation of MAPK cascade / intracellular membrane-bounded organelle /  lipid binding / regulation of DNA-templated transcription / host cell plasma membrane / positive regulation of DNA-templated transcription / lipid binding / regulation of DNA-templated transcription / host cell plasma membrane / positive regulation of DNA-templated transcription /  proteolysis / extracellular region / proteolysis / extracellular region /  membrane / membrane /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||||||||||||||||||||||||||
| Biological species |   Spodoptera frugiperda (fall armyworm) / Spodoptera frugiperda (fall armyworm) /  Priestia megaterium DSM 319 (bacteria) / Priestia megaterium DSM 319 (bacteria) /   Clostridioides difficile (bacteria) / Clostridioides difficile (bacteria) /   Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.26 Å cryo EM / Resolution: 3.26 Å | ||||||||||||||||||||||||||||||||||||
 Authors Authors | Kinsolving J / Bous J | ||||||||||||||||||||||||||||||||||||
| Funding support |  Sweden, Sweden,  Denmark, 11 items Denmark, 11 items
| ||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2024 Journal: Cell Rep / Year: 2024Title: Structural and functional insight into the interaction of Clostridioides difficile toxin B and FZD. Authors: Julia Kinsolving / Julien Bous / Pawel Kozielewicz / Sara Košenina / Rawan Shekhani / Lukas Grätz / Geoffrey Masuyer / Yuankai Wang / Pål Stenmark / Min Dong / Gunnar Schulte /   Abstract: The G protein-coupled receptors of the Frizzled (FZD) family, in particular FZD, are receptors that are exploited by Clostridioides difficile toxin B (TcdB), the major virulence factor responsible ...The G protein-coupled receptors of the Frizzled (FZD) family, in particular FZD, are receptors that are exploited by Clostridioides difficile toxin B (TcdB), the major virulence factor responsible for pathogenesis associated with Clostridioides difficile infection. We employ a live-cell assay examining the affinity between full-length FZDs and TcdB. Moreover, we present cryoelectron microscopy structures of TcdB alone and in complex with full-length FZD, which reveal that large structural rearrangements of the combined repetitive polypeptide domain are required for interaction with FZDs and other TcdB receptors, constituting a first step for receptor recognition. Furthermore, we show that bezlotoxumab, an FDA-approved monoclonal antibody to treat Clostridioides difficile infection, favors the apo-TcdB structure and thus disrupts binding with FZD. The dynamic transition between the two conformations of TcdB also governs the stability of the pore-forming region. Thus, our work provides structural and functional insight into how conformational dynamics of TcdB determine receptor binding. | ||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18374.map.gz emd_18374.map.gz | 444.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18374-v30.xml emd-18374-v30.xml emd-18374.xml emd-18374.xml | 27.6 KB 27.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18374_fsc.xml emd_18374_fsc.xml emd_18374_fsc_2.xml emd_18374_fsc_2.xml emd_18374_fsc_3.xml emd_18374_fsc_3.xml emd_18374_fsc_4.xml emd_18374_fsc_4.xml | 16.5 KB 16.4 KB 16.5 KB 16.5 KB | Display Display Display Display |  FSC data file FSC data file |
| Images |  emd_18374.png emd_18374.png | 37.2 KB | ||
| Masks |  emd_18374_msk_1.map emd_18374_msk_1.map emd_18374_msk_2.map emd_18374_msk_2.map emd_18374_msk_3.map emd_18374_msk_3.map | 476.8 MB 476.8 MB 476.8 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-18374.cif.gz emd-18374.cif.gz | 8.8 KB | ||
| Others |  emd_18374_half_map_1.map.gz emd_18374_half_map_1.map.gz emd_18374_half_map_2.map.gz emd_18374_half_map_2.map.gz | 443 MB 443 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18374 http://ftp.pdbj.org/pub/emdb/structures/EMD-18374 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18374 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18374 | HTTPS FTP |
-Related structure data
| Related structure data |  8qeoMC  8qenC  18409  18410  18411 C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_18374.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18374.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.1592 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_18374_msk_1.map emd_18374_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
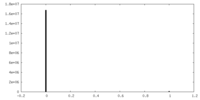
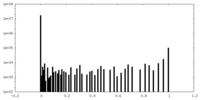
| Density Histograms |
-Mask #2
| File |  emd_18374_msk_2.map emd_18374_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
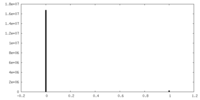
| Density Histograms |
-Mask #3
| File |  emd_18374_msk_3.map emd_18374_msk_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
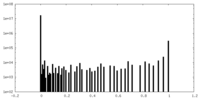
| Density Histograms |
-Half map: #2
| File | emd_18374_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_18374_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of Frizzled-7 and Clostridioides difficile toxin B
| Entire | Name: Complex of Frizzled-7 and Clostridioides difficile toxin B |
|---|---|
| Components |
|
-Supramolecule #1: Complex of Frizzled-7 and Clostridioides difficile toxin B
| Supramolecule | Name: Complex of Frizzled-7 and Clostridioides difficile toxin B type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
-Supramolecule #2: masked region--CROP domain
| Supramolecule | Name: masked region--CROP domain / type: organelle_or_cellular_component / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Priestia megaterium DSM 319 (bacteria) Priestia megaterium DSM 319 (bacteria) |
-Supramolecule #3: masked region-core domain
| Supramolecule | Name: masked region-core domain / type: organelle_or_cellular_component / ID: 3 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Priestia megaterium DSM 319 (bacteria) Priestia megaterium DSM 319 (bacteria) |
-Supramolecule #4: masked region-FZD7 CRD and leg of TcdB
| Supramolecule | Name: masked region-FZD7 CRD and leg of TcdB / type: organelle_or_cellular_component / ID: 4 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Priestia megaterium DSM 319 (bacteria) Priestia megaterium DSM 319 (bacteria) |
-Macromolecule #1: Toxin B
| Macromolecule | Name: Toxin B / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Clostridioides difficile (bacteria) Clostridioides difficile (bacteria) |
| Molecular weight | Theoretical: 273.551562 KDa |
| Recombinant expression | Organism:  Priestia megaterium DSM 319 (bacteria) Priestia megaterium DSM 319 (bacteria) |
| Sequence | String: MDKLVHLNQR GKCTMSLVNR KQLEKMANVR FRTQEDEYVA ILDALEEYHN MSENTVVEKY LKLKDINSLT DIYIDTYKKS GRNKALKKF KEYLVTEVLE LKNNNLTPVE KNLHFVWIGG QINDTAINYI NQWKDVNSDY NVNVFYDSNA FLINTLKKTV V ESAINDTL ...String: MDKLVHLNQR GKCTMSLVNR KQLEKMANVR FRTQEDEYVA ILDALEEYHN MSENTVVEKY LKLKDINSLT DIYIDTYKKS GRNKALKKF KEYLVTEVLE LKNNNLTPVE KNLHFVWIGG QINDTAINYI NQWKDVNSDY NVNVFYDSNA FLINTLKKTV V ESAINDTL ESFRENLNDP RFDYNKFFRK RMEIIYDKQK NFINYYKAQR EENPELIIDD IVKTYLSNEY SKEIDELNTY IE ESLNKIT QNSGNDVRNF EEFKNGESFN LYEQELVERW NLAAASDILR ISALKEIGGM YLDVDMLPGI QPDLFESIEK PSS VTVDFW EMTKLEAIMK YKEYIPEYTS EHFDMLDEEV QSSFESVLAS KSDKSEIFSS LGDMEASPLE VKIAFNSKGI INQG LISVK DSYCSNLIVK QIENRYKILN NSLNPAISED NDFNTTTNTF IDSIMAEANA DNGRFMMELG KYLRVGFFPD VKTTI NLSG PEAYAAAYQD LLMFKEGSMN IHLIEADLRN FEISKTNISQ STEQEMASLW SFDDARAKAQ FEEYKRNYFE GSLGED DNL DFSQNIVVDK EYLLEKISSL ARSSERGYIH YIVQLQGDKI SYEAACNLFA KTPYDSVLFQ KNIEDSEIAY YYNPGDG EI QEIDKYKIPS IISDRPKIKL TFIGHGKDEF NTDIFAGFDV DSLSTEIEAA IDLAKEDISP KSIEINLLGC NMFSYSIN V EETYPGKLLL KVKDKISELM PSISQDSIIV SANQYEVRIN SEGRRELLDH SGEWINKEES IIKDISSKEY ISFNPKENK ITVKSKNLPE LSTLLQEIRN NSNSSDIELE EKVMLTECEI NVISNIDTQI VEERIEEAKN LTSDSINYIK DEFKLIESIS DALCDLKQQ NELEDSHFIS FEDISETDEG FSIRFINKET GESIFVETEK TIFSEYANHI TEEISKIKGT IFDTVNGKLV K KVNLDTTH EVNTLNAAFF IQSLIEYNSS KESLSNLSVA MKVQVYAQLF STGLNTITDA AKVVELVSTA LDETIDLLPT LS EGLPIIA TIIDGVSLGA AIKELSETSD PLLRQEIEAK IGIMAVNLTT ATTAIITSSL GIASGFSILL VPLAGISAGI PSL VNNELV LRDKATKVVD YFKHVSLVET EGVFTLLDDK IMMPQDDLVI SEIDFNNNSI VLGKCEIWRM EGGSGHTVTD DIDH FFSAP SITYREPHLS IYDVLEVQKE ELDLSKDLMV LPNAPNRVFA WETGWTPGLR SLENDGTKLL DRIRDNYEGE FYWRY FAFI ADALITTLKP RYEDTNIRIN LDSNTRSFIV PIITTEYIRE KLSYSFYGSG GTYALSLSQY NMGINIELSE SDVWII DVD NVVRDVTIES DKIKKGDLIE GILSTLSIEE NKIILNSHEI NFSGEVNGSN GFVSLTFSIL EGINAIIEVD LLSKSYK LL ISGELKILML NSNHIQQKID YIGFNSELQK NIPYSFVDSE GKENGFINGS TKEGLFVSEL PDVVLISKVY MDDSKPSF G YYSNNLKDVK VITKDNVNIL TGYYLKDDIK ISLSLTLQDE KTIKLNSVHL DESGVAEILK FMNRKGNTNT SDSLMSFLE SMNIKSIFVN FLQSNIKFIL DANFIISGTT SIGQFEFICD ENDNIQPYFI KFNTLETNYT LYVGNRQNMI VEPNYDLDDS GDISSTVIN FSQKYLYGID SCVNKVVISP NIYTDEINIT PVYETNNTYP EVIVLDANYI NEKINVNIND LSIRYVWSND G NDFILMST SEENKVSQVK IRFVNVFKDK TLANKLSFNF SDKQDVPVSE IILSFTPSYY EDGLIGYDLG LVSLYNEKFY IN NFGMMVS GLIYINDSLY YFKPPVNNLI TGFVTVGDDK YYFNPINGGA ASIGETIIDD KNYYFNQSGV LQTGVFSTED GFK YFAPAN TLDENLEGEA IDFTGKLIID ENIYYFDDNY RGAVEWKELD GEMHYFSPET GKAFKGLNQI GDYKYYFNSD GVMQ KGFVS INDNKHYFDD SGVMKVGYTE IDGKHFYFAE NGEMQIGVFN TEDGFKYFAH HNEDLGNEEG EEISYSGILN FNNKI YYFD DSFTAVVGWK DLEDGSKYYF DEDTAEAYIG LSLINDGQYY FNDDGIMQVG FVTINDKVFY FSDSGIIESG VQNIDD NYF YIDDNGIVQI GVFDTSDGYK YFAPANTVND NIYGQAVEYS GLVRVGEDVY YFGETYTIET GWIYDMENES DKYYFNP ET KKACKGINLI DDIKYYFDEK GIMRTGLISF ENNNYYFNEN GEMQFGYINI EDKMFYFGED GVMQIGVFNT PDGFKYFA H QNTLDENFEG ESINYTGWLD LDEKRYYFTD EYIAATGSVI IDGEEYYFDP DTAQLVISEG YRPHAGLRGS HHHHHH UniProtKB: Toxin B |
-Macromolecule #2: Frizzled-7
| Macromolecule | Name: Frizzled-7 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 67.460859 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDQPYHGEK GISVPDHGFC QPISIPLCTD IAYNQTILPN LLGHTNQEDA GLEVHQFYPL VKVQCSPEL RFFLCSMYAP VCTVLDQAIP PCRSLCERAR QGCEALMNKF GFQWPERLRC ENFPVHGAGE ICVGQNTSDG S GGPGGGPT ...String: MKTIIALSYI FCLVFADYKD DDDQPYHGEK GISVPDHGFC QPISIPLCTD IAYNQTILPN LLGHTNQEDA GLEVHQFYPL VKVQCSPEL RFFLCSMYAP VCTVLDQAIP PCRSLCERAR QGCEALMNKF GFQWPERLRC ENFPVHGAGE ICVGQNTSDG S GGPGGGPT AYPTAPYLPD LPFTALPPGA SDGRGRPAFP FSCPRQLKVP PYLGYRFLGE RDCGAPCEPG RANGLMYFKE EE RRFARLW VGVWSVLCCA STLFTVLTYL VDMRRFSYPE RPIIFLSGCY FMVAVAHVAG FLLEDRAVCV ERFSDDGYRT VAQ GTKKEG CTILFMVLYF FGMASSIWWV ILSLTWFLAA GMKWGHEAIE ANSQYFHLAA WAVPAVKTIT ILAMGQVDGD LLSG VCYVG LSSVDALRGF VLAPLFVYLF IGTSFLLAGF VSLFRIRTIM KHDGTKTEKL EKLMVRIGVF SVLYTVPATI VLACY FYEQ AFREHWERTW LLQTCKSYAV PCPPGHFPPM SPDFTVFMIK YLMTMIVGIT TGFWIWSGKT LQSWRRFYHR LSHSSK GET AVSRLEVLFQ GPWSHPQFEK GGGSGGGSGG GSWSHPQFEK UniProtKB:  Frizzled-7 Frizzled-7 |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.272 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 100 mM TRIS-HCl pH 7.5 200 mM NaCl 0.002% LMNG 0.0002% CHS 0.0002% GDN | ||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.101 kPa | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.6 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.6 µm |
| Specialist optics | Energy filter - Slit width: 20 eV |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 17269 / Average exposure time: 3.0 sec. / Average electron dose: 50.453 e/Å2 Details: Images collected in super-resolution mode, faster acquisition mode, with 4 exposures per hole |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-8qeo: |
 Movie
Movie Controller
Controller













 Z
Z Y
Y X
X