[English] 日本語
 Yorodumi
Yorodumi- EMDB-17026: CryoEM Structure INO80core Hexasome complex Rvb core refinement state2 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM Structure INO80core Hexasome complex Rvb core refinement state2 | |||||||||||||||
 Map data Map data | ctINO80 Hexasome focused refinement on Rvb1/2-Ino80insert-Arp5-Ies6-Ies2 sharpened map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | ATP-dependent chromatin remodeler /  DNA BINDING PROTEIN DNA BINDING PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationDASH complex / protein transport along microtubule to mitotic spindle pole body / mitotic sister chromatid biorientation / attachment of spindle microtubules to kinetochore / Ino80 complex / attachment of mitotic spindle microtubules to kinetochore / ATP-dependent activity, acting on DNA /  helicase activity / helicase activity /  mitotic spindle / mitotic spindle /  kinetochore ...DASH complex / protein transport along microtubule to mitotic spindle pole body / mitotic sister chromatid biorientation / attachment of spindle microtubules to kinetochore / Ino80 complex / attachment of mitotic spindle microtubules to kinetochore / ATP-dependent activity, acting on DNA / kinetochore ...DASH complex / protein transport along microtubule to mitotic spindle pole body / mitotic sister chromatid biorientation / attachment of spindle microtubules to kinetochore / Ino80 complex / attachment of mitotic spindle microtubules to kinetochore / ATP-dependent activity, acting on DNA /  helicase activity / helicase activity /  mitotic spindle / mitotic spindle /  kinetochore / chromatin organization / kinetochore / chromatin organization /  DNA helicase / DNA helicase /  chromatin remodeling / chromatin remodeling /  DNA repair / DNA repair /  ATP hydrolysis activity / ATP hydrolysis activity /  ATP binding / ATP binding /  nucleus nucleusSimilarity search - Function | |||||||||||||||
| Biological species |   Thermochaetoides thermophila (fungus) Thermochaetoides thermophila (fungus) | |||||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.87 Å cryo EM / Resolution: 2.87 Å | |||||||||||||||
 Authors Authors | Zhang M / Jungblut A / Hoffmann T / Eustermann S | |||||||||||||||
| Funding support |  Germany, European Union, 4 items Germany, European Union, 4 items
| |||||||||||||||
 Citation Citation | Journal: Acta Crystallogr D Biol Crystallogr / Year: 2010 Title: Features and development of Coot. Authors: P Emsley / B Lohkamp / W G Scott / K Cowtan /  Abstract: Coot is a molecular-graphics application for model building and validation of biological macromolecules. The program displays electron-density maps and atomic models and allows model manipulations ...Coot is a molecular-graphics application for model building and validation of biological macromolecules. The program displays electron-density maps and atomic models and allows model manipulations such as idealization, real-space refinement, manual rotation/translation, rigid-body fitting, ligand search, solvation, mutations, rotamers and Ramachandran idealization. Furthermore, tools are provided for model validation as well as interfaces to external programs for refinement, validation and graphics. The software is designed to be easy to learn for novice users, which is achieved by ensuring that tools for common tasks are 'discoverable' through familiar user-interface elements (menus and toolbars) or by intuitive behaviour (mouse controls). Recent developments have focused on providing tools for expert users, with customisable key bindings, extensions and an extensive scripting interface. The software is under rapid development, but has already achieved very widespread use within the crystallographic community. The current state of the software is presented, with a description of the facilities available and of some of the underlying methods employed. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17026.map.gz emd_17026.map.gz | 5.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17026-v30.xml emd-17026-v30.xml emd-17026.xml emd-17026.xml | 34.6 KB 34.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17026_fsc.xml emd_17026_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_17026.png emd_17026.png | 87 KB | ||
| Others |  emd_17026_additional_1.map.gz emd_17026_additional_1.map.gz emd_17026_half_map_1.map.gz emd_17026_half_map_1.map.gz emd_17026_half_map_2.map.gz emd_17026_half_map_2.map.gz | 140.6 MB 140.9 MB 140.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17026 http://ftp.pdbj.org/pub/emdb/structures/EMD-17026 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17026 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17026 | HTTPS FTP |
-Related structure data
| Related structure data |  8oorMC  8oo7C  8oo9C  8ooaC  8oocC  8oofC  8ookC  8oopC  8oosC  8ootC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17026.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17026.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ctINO80 Hexasome focused refinement on Rvb1/2-Ino80insert-Arp5-Ies6-Ies2 sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.822 Å | ||||||||||||||||||||||||||||||||||||
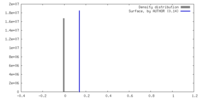
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: ctINO80 Hexasome Core Map unsharpened
| File | emd_17026_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ctINO80 Hexasome Core Map unsharpened | ||||||||||||
| Projections & Slices |
| ||||||||||||
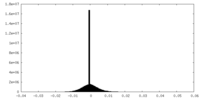
| Density Histograms |
-Half map: ctINO80 Hexasome Core Map half map 1
| File | emd_17026_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ctINO80 Hexasome Core Map half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
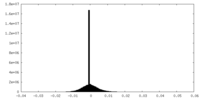
| Density Histograms |
-Half map: ctINO80 Hexasome Core Map half map 2
| File | emd_17026_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ctINO80 Hexasome Core Map half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
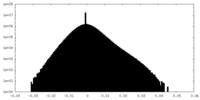
| Density Histograms |
- Sample components
Sample components
+Entire : INO80 core module in complex with hexasome
+Supramolecule #1: INO80 core module in complex with hexasome
+Macromolecule #1: RuvB-like protein 1
+Macromolecule #2: RuvB-like protein 2
+Macromolecule #3: Chromatin-remodeling ATPase Ino80
+Macromolecule #4: Ino eighty subunit 2
+Macromolecule #5: Chromatin-remodeling complex subunit IES6
+Macromolecule #6: Actin-related protein 5
+Macromolecule #7: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #8: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #9: MAGNESIUM ION
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.88 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 30mM HEPES, pH7.5 50mM NaCl 0.25mM CaCl2 0.25mM DTT 2mM ADP 3.3mM MgCl2 10mM NaF 2mM AlCl3 0.05% octyl-beta-glucoside |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 15 sec. / Details: 10% Oxygene 90% Argon |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: wait time of 5s, blot force at 3, and a blot time of 2s with Whatman blotting paper (Cytiva, CAT No. 10311807). |
| Details | 11-subunit ctINO80 reconstituted with hexasome |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 15384 / Average electron dose: 50.36 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)