[English] 日本語
 Yorodumi
Yorodumi- EMDB-17007: CryoEM Structure INO80core Hexasome complex ATPase-DNA refinement... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM Structure INO80core Hexasome complex ATPase-DNA refinement state1 | |||||||||||||||
 Map data Map data | ctINO80-hexasome ATPase-DNA focused refinement sharpened map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | ATP-dependent chromatin remodeler /  DNA BINDING PROTEIN DNA BINDING PROTEIN | |||||||||||||||
| Biological species |   Thermochaetoides thermophila (fungus) / synthetic construct (others) Thermochaetoides thermophila (fungus) / synthetic construct (others) | |||||||||||||||
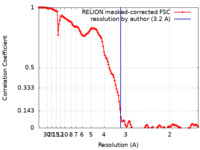
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | |||||||||||||||
 Authors Authors | Zhang M / Jungblut A / Hoffmann T / Eustermann S | |||||||||||||||
| Funding support |  Germany, European Union, 4 items Germany, European Union, 4 items
| |||||||||||||||
 Citation Citation | Journal: Acta Crystallogr D Biol Crystallogr / Year: 2010 Title: Features and development of Coot. Authors: P Emsley / B Lohkamp / W G Scott / K Cowtan /  Abstract: Coot is a molecular-graphics application for model building and validation of biological macromolecules. The program displays electron-density maps and atomic models and allows model manipulations ...Coot is a molecular-graphics application for model building and validation of biological macromolecules. The program displays electron-density maps and atomic models and allows model manipulations such as idealization, real-space refinement, manual rotation/translation, rigid-body fitting, ligand search, solvation, mutations, rotamers and Ramachandran idealization. Furthermore, tools are provided for model validation as well as interfaces to external programs for refinement, validation and graphics. The software is designed to be easy to learn for novice users, which is achieved by ensuring that tools for common tasks are 'discoverable' through familiar user-interface elements (menus and toolbars) or by intuitive behaviour (mouse controls). Recent developments have focused on providing tools for expert users, with customisable key bindings, extensions and an extensive scripting interface. The software is under rapid development, but has already achieved very widespread use within the crystallographic community. The current state of the software is presented, with a description of the facilities available and of some of the underlying methods employed. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17007.map.gz emd_17007.map.gz | 8.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17007-v30.xml emd-17007-v30.xml emd-17007.xml emd-17007.xml | 28.6 KB 28.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17007_fsc.xml emd_17007_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_17007.png emd_17007.png | 53.9 KB | ||
| Others |  emd_17007_additional_1.map.gz emd_17007_additional_1.map.gz emd_17007_half_map_1.map.gz emd_17007_half_map_1.map.gz emd_17007_half_map_2.map.gz emd_17007_half_map_2.map.gz | 140.4 MB 140.6 MB 140.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17007 http://ftp.pdbj.org/pub/emdb/structures/EMD-17007 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17007 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17007 | HTTPS FTP |
-Related structure data
| Related structure data |  8oo9MC  8oo7C  8ooaC  8oocC  8oofC  8ookC  8oopC  8oorC  8oosC  8ootC C: citing same article ( M: atomic model generated by this map |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_17007.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17007.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ctINO80-hexasome ATPase-DNA focused refinement sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.822 Å | ||||||||||||||||||||||||||||||||||||
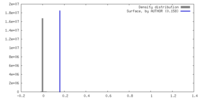
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: unsharpened map ctINO80-hexasome ATPase-DNA focused refinement
| File | emd_17007_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened map ctINO80-hexasome ATPase-DNA focused refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
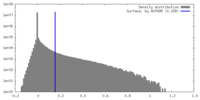
| Density Histograms |
-Half map: half map 1 ctINO80-hexasome ATPase-DNA focused refinement
| File | emd_17007_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 ctINO80-hexasome ATPase-DNA focused refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
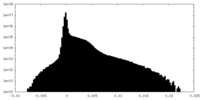
| Density Histograms |
-Half map: half map 2 ctINO80-hexasome ATPase-DNA focused refinement
| File | emd_17007_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 ctINO80-hexasome ATPase-DNA focused refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
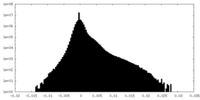
| Density Histograms |
- Sample components
Sample components
-Entire : INO80 core module in complex with hexasome
| Entire | Name: INO80 core module in complex with hexasome |
|---|---|
| Components |
|
-Supramolecule #1: INO80 core module in complex with hexasome
| Supramolecule | Name: INO80 core module in complex with hexasome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 Details: 11-subunit ct INO80 contains two modules (core and Arp8 module) Each module was picked and analyzed separately The core module + hexasome has an overall weight of 0.861MDa The 11-subunit ct ...Details: 11-subunit ct INO80 contains two modules (core and Arp8 module) Each module was picked and analyzed separately The core module + hexasome has an overall weight of 0.861MDa The 11-subunit ct INO80 + hexasome has an overall weight of 1.1MDa Ino80, Ies2, Ies6, Ies4,Arp6, Rvb1, Rvb2, Arp8, Arp4, Actin, Taf14 Hexasome DNA, 2xH3, 2xH4, H2A, H2B |
|---|---|
| Source (natural) | Organism:   Thermochaetoides thermophila (fungus) Thermochaetoides thermophila (fungus) |
| Molecular weight | Theoretical: 861 KDa |
-Macromolecule #1: Chromatin-remodeling ATPase INO80
| Macromolecule | Name: Chromatin-remodeling ATPase INO80 / type: protein_or_peptide / ID: 1 / Details: Ino80 N-terminal truncation C-terminal 2xFlagTag / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Thermochaetoides thermophila (fungus) Thermochaetoides thermophila (fungus) |
| Molecular weight | Theoretical: 130.887656 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: LELKFQSKGY NQIYDQIWRD LARKDVSKVF RLATDSYATK ASNLKKTAIL ASKEAKRWQL RTNKGTKDLQ ARAKRVMRDM MGFWKRNER EERDLRKAAE RLELENARKE EADREAARQR RKLNFLISQT ELYSHFISKK IKTHEVERST DHPDVATDEK D KIPEPTLN ...String: LELKFQSKGY NQIYDQIWRD LARKDVSKVF RLATDSYATK ASNLKKTAIL ASKEAKRWQL RTNKGTKDLQ ARAKRVMRDM MGFWKRNER EERDLRKAAE RLELENARKE EADREAARQR RKLNFLISQT ELYSHFISKK IKTHEVERST DHPDVATDEK D KIPEPTLN INVPEPTGPI APKVTDFNSL DFDNEDESAL QAAAMANAQN AIAEAQKKAR EFNKDETKLD EDGEMNFQHP EL TEFEVAQ PKLLNCQLKE YQLKGLNWLV NLYEQGINGI LADEMGLGKT VQSISVMAYL AERYDIWGPF LVVAPASTLH NWQ QEVSKF VPDFKVLPYW GTAADRKVLR KFWDRKHTTY KKDSPFHVMI TSYQLVVSDV AYFQKMKWQY MILDEAQAIK SSQS SRWKC LLGFHCRNRL LLTGTPIQNN MQELWALLHF IMPSLFDSHD EFSEWFSKDI ESHAQSNTKL NEDQLKRLHM ILKPF MLRR VKKHVQKELG DKIEIDVFCE LSYRQRAMYQ SLRNQISIMD LIEKATVGDN EDSATLMNLV MQFRKVCNHP DLFERA DTS SPFFCGHFAE TGSFLREGTN VALGYSTRSL VEYRLPRLIW CDGGRLDKPG PGNLVAGFRS KYLNHMMNIW TPENIRS SL EGIENFTWLR FVDTSLQEAY RASHTDVFAR AVDLASKQNR LGHMQIVYDE PEDKKWTPVH ALFQICEREN PKAVAEIT T EGVLRDLMNI ARVKYRELGL CRLEKAARPR ASAPPIEVVC DSRSAVIERE NIMFHPAMRK ALFGPTPSEI KEASFGPRP VTLYPPRALL PAPDHDKQRF TNITVPSMAR FVTDSGKLAK LDELLRELKE GGHRVLLYFQ MTRMIDLMEE YLTYRNYKYC RLDGSTKLE DRRDTVADFQ TRPEIFIFLL STRAGGLGIN LTTADTVIFY DSDWNPTIDS QAMDRAHRLG QTKQVTVYRL I TRGTIEER IRKRALQKEE VQRVVITGTG SVDFSGRRPP ENRNRDIAMW LADDEQAEMI ERREKELIES GEYDKIMQQR RK GGKRKRG AANGDTVPSL EDMYHEGEGH FDDNKGSGAA TPVDADSLGR GGKRKKAGGS KKAKTTKQRL AIADGEIDID YKD DDDKGT DYKDDDDK |
-Macromolecule #2: DNA strand 1
| Macromolecule | Name: DNA strand 1 / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 69.527195 KDa |
| Sequence | String: (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA) (DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA)(DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA) (DG) (DC)(DA)(DA)(DG)(DC)(DT) ...String: (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA) (DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA)(DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA) (DG) (DC)(DA)(DA)(DG)(DC)(DT)(DC)(DT) (DA)(DG)(DC)(DA)(DC)(DC)(DG)(DC)(DT)(DT) (DA)(DA) (DA)(DC)(DG)(DC)(DA)(DC)(DG) (DT)(DA)(DC)(DG)(DC)(DG)(DC)(DT)(DG)(DT) (DC)(DC)(DC) (DC)(DC)(DG)(DC)(DG)(DT) (DT)(DT)(DT)(DA)(DA)(DC)(DC)(DG)(DC)(DC) (DA)(DA)(DG)(DG) (DG)(DG)(DA)(DT)(DT) (DA)(DC)(DT)(DC)(DC)(DC)(DT)(DA)(DG)(DT) (DC)(DT)(DC)(DC)(DA) (DG)(DG)(DC)(DA) (DC)(DG)(DT)(DG)(DT)(DC)(DA)(DG)(DA)(DT) (DA)(DT)(DA)(DT)(DA)(DC) (DA)(DT)(DC) (DC)(DT)(DG)(DT)(DG)(DC)(DA)(DT)(DG)(DT) (DA)(DT)(DT)(DG)(DA)(DA)(DC) (DA)(DG) (DC)(DG)(DA)(DC)(DC)(DT)(DT)(DG)(DC)(DC) (DG)(DG)(DT)(DG)(DC)(DC)(DA)(DG) (DT) (DC)(DG)(DG)(DA)(DT)(DA)(DG)(DT)(DG)(DT) (DT)(DC)(DC)(DG)(DA)(DG)(DC)(DT)(DC) (DC)(DC)(DA)(DC)(DT)(DC)(DT)(DA)(DG)(DA) (DG)(DG)(DA)(DT)(DC)(DC)(DC)(DC)(DG)(DG) (DG)(DT)(DA)(DC)(DC)(DG) |
-Macromolecule #3: DNA strand 2
| Macromolecule | Name: DNA strand 2 / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 70.043562 KDa |
| Sequence | String: (DC)(DG)(DG)(DT)(DA)(DC)(DC)(DC)(DG)(DG) (DG)(DG)(DA)(DT)(DC)(DC)(DT)(DC)(DT)(DA) (DG)(DA)(DG)(DT)(DG)(DG)(DG)(DA)(DG) (DC)(DT)(DC)(DG)(DG)(DA)(DA)(DC)(DA)(DC) (DT) (DA)(DT)(DC)(DC)(DG)(DA) ...String: (DC)(DG)(DG)(DT)(DA)(DC)(DC)(DC)(DG)(DG) (DG)(DG)(DA)(DT)(DC)(DC)(DT)(DC)(DT)(DA) (DG)(DA)(DG)(DT)(DG)(DG)(DG)(DA)(DG) (DC)(DT)(DC)(DG)(DG)(DA)(DA)(DC)(DA)(DC) (DT) (DA)(DT)(DC)(DC)(DG)(DA)(DC)(DT) (DG)(DG)(DC)(DA)(DC)(DC)(DG)(DG)(DC)(DA) (DA)(DG) (DG)(DT)(DC)(DG)(DC)(DT)(DG) (DT)(DT)(DC)(DA)(DA)(DT)(DA)(DC)(DA)(DT) (DG)(DC)(DA) (DC)(DA)(DG)(DG)(DA)(DT) (DG)(DT)(DA)(DT)(DA)(DT)(DA)(DT)(DC)(DT) (DG)(DA)(DC)(DA) (DC)(DG)(DT)(DG)(DC) (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DC)(DT)(DA) (DG)(DG)(DG)(DA)(DG) (DT)(DA)(DA)(DT) (DC)(DC)(DC)(DC)(DT)(DT)(DG)(DG)(DC)(DG) (DG)(DT)(DT)(DA)(DA)(DA) (DA)(DC)(DG) (DC)(DG)(DG)(DG)(DG)(DG)(DA)(DC)(DA)(DG) (DC)(DG)(DC)(DG)(DT)(DA)(DC) (DG)(DT) (DG)(DC)(DG)(DT)(DT)(DT)(DA)(DA)(DG)(DC) (DG)(DG)(DT)(DG)(DC)(DT)(DA)(DG) (DA) (DG)(DC)(DT)(DT)(DG)(DC)(DT)(DA)(DC)(DG) (DA)(DC)(DC)(DA)(DA)(DT)(DT)(DG)(DA) (DG)(DC)(DG)(DG)(DC)(DC)(DT)(DC)(DG)(DG) (DC)(DA)(DC)(DC)(DG)(DG)(DG)(DA)(DT)(DT) (DC)(DT)(DC)(DC)(DA)(DG) |
-Macromolecule #4: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 1 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: TETRAFLUOROALUMINATE ION
| Macromolecule | Name: TETRAFLUOROALUMINATE ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: ALF |
|---|---|
| Molecular weight | Theoretical: 102.975 Da |
| Chemical component information |  ChemComp-ALF: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.88 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 30mM HEPES, pH7.5 50mM NaCl 0.25mM CaCl2 0.25mM DTT 2mM ADP 3.3mM MgCl2 10mM NaF 2mM AlCl3 0.05% octyl-beta-glucoside |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 15 sec. / Details: 10% Oxygene 90% Argone |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: wait time of 5s, blot force at 3, and a blot time of 2s with Whatman blotting paper (Cytiva, CAT No. 10311807). |
| Details | 11-subunit ctINO80 reconstituted with hexasome |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 15384 / Average electron dose: 50.36 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)