+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HB3VAR03 apo headstructure (PfEMP1 A) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  Plasmodium falciparum / Cerebral Malaria / Plasmodium falciparum / Cerebral Malaria /  PfEMP1 / EPCR / PfEMP1 / EPCR /  Cell adhesion Cell adhesion | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |   Plasmodium falciparum HB3 (eukaryote) Plasmodium falciparum HB3 (eukaryote) | |||||||||
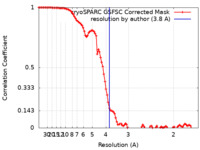
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.8 Å cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Raghavan SSR / Lavstsen T / Wang KT | |||||||||
| Funding support |  Denmark, 2 items Denmark, 2 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2023 Journal: Structure / Year: 2023Title: Endothelial protein C receptor binding induces conformational changes to severe malaria-associated group A PfEMP1. Authors: Sai Sundar Rajan Raghavan / Louise Turner / Rasmus W Jensen / Nicolai Tidemand Johansen / Daniel Skjold Jensen / Pontus Gourdon / Jinqiu Zhang / Yong Wang / Thor Grundtvig Theander / Kaituo ...Authors: Sai Sundar Rajan Raghavan / Louise Turner / Rasmus W Jensen / Nicolai Tidemand Johansen / Daniel Skjold Jensen / Pontus Gourdon / Jinqiu Zhang / Yong Wang / Thor Grundtvig Theander / Kaituo Wang / Thomas Lavstsen /   Abstract: Severe Plasmodium falciparum malaria infections are caused by microvascular sequestration of parasites binding to the human endothelial protein C receptor (EPCR) via the multi-domain P. falciparum ...Severe Plasmodium falciparum malaria infections are caused by microvascular sequestration of parasites binding to the human endothelial protein C receptor (EPCR) via the multi-domain P. falciparum erythrocyte membrane protein 1 (PfEMP1) adhesion ligands. Using cryogenic electron microscopy (Cryo-EM) and PfEMP1 sequence diversity analysis, we found that group A PfEMP1 CIDRα1 domains interact with the adjacent DBLα1 domain through central, conserved residues of the EPCR-binding site to adopt a compact conformation. Upon EPCR binding, the DBLα1 domain is displaced, and the EPCR-binding helix of CIDRα1 is turned, kinked, and twisted to reach a rearranged, stable EPCR-bound conformation. The unbound conformation and the required transition to the EPCR-bound conformation may represent a conformational masking mechanism of immune evasion for the PfEMP1 family. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16415.map.gz emd_16415.map.gz | 52.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16415-v30.xml emd-16415-v30.xml emd-16415.xml emd-16415.xml | 14.8 KB 14.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16415_fsc.xml emd_16415_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_16415.png emd_16415.png | 201.9 KB | ||
| Filedesc metadata |  emd-16415.cif.gz emd-16415.cif.gz | 6 KB | ||
| Others |  emd_16415_half_map_1.map.gz emd_16415_half_map_1.map.gz emd_16415_half_map_2.map.gz emd_16415_half_map_2.map.gz | 95.7 MB 95.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16415 http://ftp.pdbj.org/pub/emdb/structures/EMD-16415 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16415 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16415 | HTTPS FTP |
-Related structure data
| Related structure data |  8c3yMC  8c44C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_16415.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16415.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.832 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_16415_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_16415_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Multidomain architecture of PfEMP1 A
| Entire | Name: Multidomain architecture of PfEMP1 A |
|---|---|
| Components |
|
-Supramolecule #1: Multidomain architecture of PfEMP1 A
| Supramolecule | Name: Multidomain architecture of PfEMP1 A / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Plasmodium falciparum HB3 (eukaryote) Plasmodium falciparum HB3 (eukaryote) |
| Molecular weight | Theoretical: 145 KDa |
-Macromolecule #1: PfEMP1
| Macromolecule | Name: PfEMP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Plasmodium falciparum HB3 (eukaryote) Plasmodium falciparum HB3 (eukaryote) |
| Molecular weight | Theoretical: 145.128734 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MASSASKFSK IVVGNETHKS ARNVLEGFAK DIKGKASIDA EKHAYSLKGN LKDAKFNHDF FKIKSDMPGN PCYLDFAFHS NTPGNQREY RHPCARSMNK NLFNLEGAVC TNSKIKGNEE KINGAGACAP YRRRHICDLN LEHIDVHNVQ NIHDLLGNVL V TAKYEGES ...String: MASSASKFSK IVVGNETHKS ARNVLEGFAK DIKGKASIDA EKHAYSLKGN LKDAKFNHDF FKIKSDMPGN PCYLDFAFHS NTPGNQREY RHPCARSMNK NLFNLEGAVC TNSKIKGNEE KINGAGACAP YRRRHICDLN LEHIDVHNVQ NIHDLLGNVL V TAKYEGES IVEKHPNRGS SEVCTALARS FADIGDIIRG KDLYLGHEQG NNKLEARLKT IFQNIKNKNK SPLDKLSLEQ VR EYWWALN REDVWKALTC FADGSEEYFI QSSDKEHSFS SEYCGHEQGN VPTNLDYVPQ FLRWFDEWAD DFCRIKKIKL ENV KNACRD EKKRKYCSLN GFDCTQTIWK KGVLHRSNEC TGCLVKCNPY EIWLGNQREA FRKQKEKYEN EIKTYVHDTG ISNS NINNE YYKEFYKILK NNNYETANEF IKLLNEGRYC NKKEKIEEEE DIDFTNTNEK GTFYRSDYCQ VCPDCGVECK NETCT PKTV IYPDCGKNEK YEPPGDAKNT EINVINSGDK EGYIFEKLSE FCTNENNENG KNYEQWKCYY DNKKNNNKCK MEINIA NSK LKNKITSFDE FFDFWVRKLL IDTIKWETEL TYCINNTDVT DCNKCNKNCV CFDKWVKQKE DEWTNIMKLF TNKHDIP KK YYLNINDLFD SFFFQVIYKF NEGEAKWNEL KENLKKQIAS SKANNGTKDS EAAIKVLFNH IKEIATICKD NNTNEGCD P SVDSKTNSCG KNTKAGSDKV ISVKQIAQYY KRIAHKQLNE RGSRSALKGD ASKGTYKKNG TPSNLKEICE ITAKHSNDS RRDGEPCTGK DGGQVRVRTK IGTPWTKIVE INKTSYKEVF LPPRRQHMCT SNLEHLNTGN KGLKDGKLAI HSLLGDVLLA AKEQANFIK NKYKRQKASN GFKDKGTICR AIRYSYADLG DIIKGTDLWE ANPGEKNTQR RLKTVFGIIK KNMPGIKDNQ K YKDDEKNN PPYKLLREDW WEANRDQVWQ AMKCAMKNGI TCGSSDHTPL DDYIPQKLRW LTEWAEWYCK AQSKEYEKLK EK CKECKGN DQCTQDTPDC EKCKAACKKY GKNIKTWEDQ WKVISSKYKE LYKQAEIYAG NGGPGYYNTK VQEEDKPVVD FLY NLYLQN GGKKGPPPDT HPSKSVTAPL KQVATVDTPS TVYSTPEGYI HQEAAMDCKQ QHVFCDDNSG GKDDNKQYAF RHQP HDYDE ALRCDQRDKP PPESKKVEKA KKEKDENDDG GSHHHHHHGG GSAHIVMVDA YKPTK UniProtKB:  PfEMP1 PfEMP1 |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.2 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.2 µm |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 48.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z
Z Y
Y X
X