+ Open data
Open data
- Basic information
Basic information
| Entry | 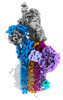 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of C5b8-CD59 | |||||||||
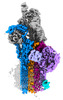
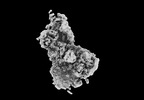
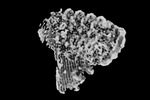
 Map data Map data | CryoEM reconstruction of C5b8-CD59. | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of activation of membrane attack complex / Terminal pathway of complement /  membrane attack complex / complement binding / membrane attack complex / complement binding /  regulation of complement-dependent cytotoxicity / regulation of complement-dependent cytotoxicity /  regulation of complement activation / Activation of C3 and C5 / negative regulation of macrophage chemotaxis / Cargo concentration in the ER / regulation of complement activation / Activation of C3 and C5 / negative regulation of macrophage chemotaxis / Cargo concentration in the ER /  complement activation, alternative pathway ...negative regulation of activation of membrane attack complex / Terminal pathway of complement / complement activation, alternative pathway ...negative regulation of activation of membrane attack complex / Terminal pathway of complement /  membrane attack complex / complement binding / membrane attack complex / complement binding /  regulation of complement-dependent cytotoxicity / regulation of complement-dependent cytotoxicity /  regulation of complement activation / Activation of C3 and C5 / negative regulation of macrophage chemotaxis / Cargo concentration in the ER / regulation of complement activation / Activation of C3 and C5 / negative regulation of macrophage chemotaxis / Cargo concentration in the ER /  complement activation, alternative pathway / complement activation, alternative pathway /  complement activation / complement activation /  chemokine activity / COPII-mediated vesicle transport / chemokine activity / COPII-mediated vesicle transport /  retinol binding / retinol binding /  endopeptidase inhibitor activity / tertiary granule membrane / positive regulation of vascular endothelial growth factor production / specific granule membrane / COPI-mediated anterograde transport / endopeptidase inhibitor activity / tertiary granule membrane / positive regulation of vascular endothelial growth factor production / specific granule membrane / COPI-mediated anterograde transport /  complement activation, classical pathway / positive regulation of chemokine production / complement activation, classical pathway / positive regulation of chemokine production /  transport vesicle / endoplasmic reticulum-Golgi intermediate compartment membrane / Peptide ligand-binding receptors / transport vesicle / endoplasmic reticulum-Golgi intermediate compartment membrane / Peptide ligand-binding receptors /  Regulation of Complement cascade / ER to Golgi transport vesicle membrane / Regulation of Complement cascade / ER to Golgi transport vesicle membrane /  chemotaxis / positive regulation of immune response / chemotaxis / positive regulation of immune response /  extracellular vesicle / extracellular vesicle /  blood coagulation / G alpha (i) signalling events / blood microparticle / killing of cells of another organism / in utero embryonic development / vesicle / cell surface receptor signaling pathway / blood coagulation / G alpha (i) signalling events / blood microparticle / killing of cells of another organism / in utero embryonic development / vesicle / cell surface receptor signaling pathway /  immune response / immune response /  inflammatory response / G protein-coupled receptor signaling pathway / external side of plasma membrane / inflammatory response / G protein-coupled receptor signaling pathway / external side of plasma membrane /  Golgi membrane / Golgi membrane /  signaling receptor binding / signaling receptor binding /  focal adhesion / focal adhesion /  innate immune response / Neutrophil degranulation / protein-containing complex binding / endoplasmic reticulum membrane / innate immune response / Neutrophil degranulation / protein-containing complex binding / endoplasmic reticulum membrane /  cell surface / cell surface /  extracellular space / extracellular exosome / extracellular region / extracellular space / extracellular exosome / extracellular region /  membrane / membrane /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Escherichia coli (E. coli) / Escherichia coli (E. coli) /   human (human) human (human) | |||||||||
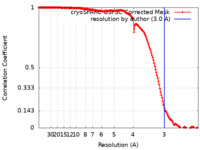
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.0 Å cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Bubeck D / Couves EC / Gardner S | |||||||||
| Funding support | European Union,  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis for membrane attack complex inhibition by CD59. Authors: Emma C Couves / Scott Gardner / Tomas B Voisin / Jasmine K Bickel / Phillip J Stansfeld / Edward W Tate / Doryen Bubeck /  Abstract: CD59 is an abundant immuno-regulatory receptor that protects human cells from damage during complement activation. Here we show how the receptor binds complement proteins C8 and C9 at the membrane to ...CD59 is an abundant immuno-regulatory receptor that protects human cells from damage during complement activation. Here we show how the receptor binds complement proteins C8 and C9 at the membrane to prevent insertion and polymerization of membrane attack complex (MAC) pores. We present cryo-electron microscopy structures of two inhibited MAC precursors known as C5b8 and C5b9. We discover that in both complexes, CD59 binds the pore-forming β-hairpins of C8 to form an intermolecular β-sheet that prevents membrane perforation. While bound to C8, CD59 deflects the cascading C9 β-hairpins, rerouting their trajectory into the membrane. Preventing insertion of C9 restricts structural transitions of subsequent monomers and indirectly halts MAC polymerization. We combine our structural data with cellular assays and molecular dynamics simulations to explain how the membrane environment impacts the dual roles of CD59 in controlling pore formation of MAC, and as a target of bacterial virulence factors which hijack CD59 to lyse human cells. #1:  Journal: Acta Crystallogr., Sect. D: Biol. Crystallogr. / Year: 2018 Journal: Acta Crystallogr., Sect. D: Biol. Crystallogr. / Year: 2018Title: Real-space refinement in PHENIX for cryo-EM and crystallography Authors: Afonine PV / Poon BK / Read RJ / Sobolev OV / Terwilliger TC / Urzhumtsev A / Adams PD | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15779.map.gz emd_15779.map.gz | 281.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15779-v30.xml emd-15779-v30.xml emd-15779.xml emd-15779.xml | 30.5 KB 30.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15779_fsc.xml emd_15779_fsc.xml | 14.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_15779.png emd_15779.png | 100.9 KB | ||
| Masks |  emd_15779_msk_1.map emd_15779_msk_1.map | 343 MB |  Mask map Mask map | |
| Others |  emd_15779_half_map_1.map.gz emd_15779_half_map_1.map.gz emd_15779_half_map_2.map.gz emd_15779_half_map_2.map.gz | 318.7 MB 318.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15779 http://ftp.pdbj.org/pub/emdb/structures/EMD-15779 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15779 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15779 | HTTPS FTP |
-Related structure data
| Related structure data |  8b0fMC  8b0gC  8b0hC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15779.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15779.map.gz / Format: CCP4 / Size: 343 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM reconstruction of C5b8-CD59. | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.171 Å | ||||||||||||||||||||
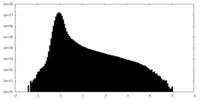
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15779_msk_1.map emd_15779_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
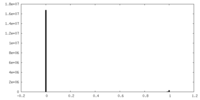
| Density Histograms |
-Half map: CryoEM reconstruction of C5b8-CD59.
| File | emd_15779_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM reconstruction of C5b8-CD59. | ||||||||||||
| Projections & Slices |
| ||||||||||||
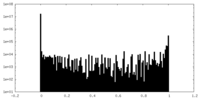
| Density Histograms |
-Half map: CryoEM reconstruction of C5b8-CD59.
| File | emd_15779_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM reconstruction of C5b8-CD59. | ||||||||||||
| Projections & Slices |
| ||||||||||||
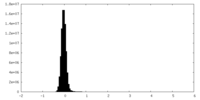
| Density Histograms |
- Sample components
Sample components
+Entire : C5b8-CD59
+Supramolecule #1: C5b8-CD59
+Supramolecule #2: Complement components C5, C6, C7 and C8
+Supramolecule #3: CD59 glycoprotein
+Macromolecule #1: Complement C5
+Macromolecule #2: Complement component C6
+Macromolecule #3: Complement component C7
+Macromolecule #4: Complement component C8 beta chain
+Macromolecule #5: Complement component C8 alpha chain
+Macromolecule #6: Complement component C8 gamma chain
+Macromolecule #7: CD59 glycoprotein
+Macromolecule #10: CALCIUM ION
+Macromolecule #11: 2-acetamido-2-deoxy-beta-D-glucopyranose
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: GRAPHENE OXIDE / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: NITROGEN |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 294 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.9 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.9 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 2 / Number real images: 12805 / Average exposure time: 9.8 sec. / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Details | Initial rigid body fits were performed using UCSF Chimera and UCSF ChimeraX. Carbohydrates were added using Coot. Models were refined iteratively using Isolde and Phenix Real Space Refine. |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 87 |
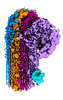
| Output model |  PDB-8b0f: |
 Movie
Movie Controller
Controller



























 Z
Z Y
Y X
X