+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Sodium pumping NADH-quinone oxidoreductase with substrate Q2 | |||||||||
 Map data Map data | NQR with substrate Q2 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  quinone / quinone /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information NADH:ubiquinone reductase (Na+-transporting) / Gram-negative-bacterium-type cell wall / oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor / sodium ion transport / respiratory electron transport chain / transmembrane transport / 2 iron, 2 sulfur cluster binding / FMN binding / NADH:ubiquinone reductase (Na+-transporting) / Gram-negative-bacterium-type cell wall / oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor / sodium ion transport / respiratory electron transport chain / transmembrane transport / 2 iron, 2 sulfur cluster binding / FMN binding /  electron transfer activity / electron transfer activity /  metal ion binding / metal ion binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||
| Biological species |   Vibrio cholerae (bacteria) Vibrio cholerae (bacteria) | |||||||||
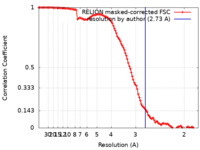
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.73 Å cryo EM / Resolution: 2.73 Å | |||||||||
 Authors Authors | Hau J-L / Kaltwasser S / Vonck J / Fritz G / Steuber J | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Conformational coupling of redox-driven Na-translocation in Vibrio cholerae NADH:quinone oxidoreductase. Authors: Jann-Louis Hau / Susann Kaltwasser / Valentin Muras / Marco S Casutt / Georg Vohl / Björn Claußen / Wojtek Steffen / Alexander Leitner / Eckhard Bill / George E Cutsail / Serena DeBeer / ...Authors: Jann-Louis Hau / Susann Kaltwasser / Valentin Muras / Marco S Casutt / Georg Vohl / Björn Claußen / Wojtek Steffen / Alexander Leitner / Eckhard Bill / George E Cutsail / Serena DeBeer / Janet Vonck / Julia Steuber / Günter Fritz /   Abstract: In the respiratory chain, NADH oxidation is coupled to ion translocation across the membrane to build up an electrochemical gradient. In the human pathogen Vibrio cholerae, the sodium-pumping NADH: ...In the respiratory chain, NADH oxidation is coupled to ion translocation across the membrane to build up an electrochemical gradient. In the human pathogen Vibrio cholerae, the sodium-pumping NADH:quinone oxidoreductase (Na-NQR) generates a sodium gradient by a so far unknown mechanism. Here we show that ion pumping in Na-NQR is driven by large conformational changes coupling electron transfer to ion translocation. We have determined a series of cryo-EM and X-ray structures of the Na-NQR that represent snapshots of the catalytic cycle. The six subunits NqrA, B, C, D, E, and F of Na-NQR harbor a unique set of cofactors that shuttle the electrons from NADH twice across the membrane to quinone. The redox state of a unique intramembranous [2Fe-2S] cluster orchestrates the movements of subunit NqrC, which acts as an electron transfer switch. We propose that this switching movement controls the release of Na from a binding site localized in subunit NqrB. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15090.map.gz emd_15090.map.gz | 117.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15090-v30.xml emd-15090-v30.xml emd-15090.xml emd-15090.xml | 27.8 KB 27.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15090_fsc.xml emd_15090_fsc.xml | 11.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_15090.png emd_15090.png | 218.3 KB | ||
| Masks |  emd_15090_msk_1.map emd_15090_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15090.cif.gz emd-15090.cif.gz | 8.4 KB | ||
| Others |  emd_15090_half_map_1.map.gz emd_15090_half_map_1.map.gz emd_15090_half_map_2.map.gz emd_15090_half_map_2.map.gz | 98.7 MB 98.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15090 http://ftp.pdbj.org/pub/emdb/structures/EMD-15090 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15090 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15090 | HTTPS FTP |
-Related structure data
| Related structure data |  8a1vMC  8a1tC  8a1uC  8a1wC  8a1xC  8a1yC  8acwC  8acyC  8ad3C  8ad4C  8ad5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15090.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15090.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | NQR with substrate Q2 | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.9125 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15090_msk_1.map emd_15090_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_15090_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_15090_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : NQR complex with substrate Q2
+Supramolecule #1: NQR complex with substrate Q2
+Macromolecule #1: Na(+)-translocating NADH-quinone reductase subunit A
+Macromolecule #2: Na(+)-translocating NADH-quinone reductase subunit B
+Macromolecule #3: Na(+)-translocating NADH-quinone reductase subunit C
+Macromolecule #4: Na(+)-translocating NADH-quinone reductase subunit D
+Macromolecule #5: Na(+)-translocating NADH-quinone reductase subunit E
+Macromolecule #6: Na(+)-translocating NADH-quinone reductase subunit F
+Macromolecule #7: RIBOFLAVIN
+Macromolecule #8: 1,2-Distearoyl-sn-glycerophosphoethanolamine
+Macromolecule #9: DODECYL-BETA-D-MALTOSIDE
+Macromolecule #10: UBIQUINONE-2
+Macromolecule #11: FLAVIN MONONUCLEOTIDE
+Macromolecule #12: SODIUM ION
+Macromolecule #13: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #14: FLAVIN-ADENINE DINUCLEOTIDE
+Macromolecule #15: water
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 7.6 |
| Grid | Model: C-flat-1.2/1.3 / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 90 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 276 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 191781 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 165000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 165000 |
| Specialist optics | Energy filter - Name: TFS Selectris X / Energy filter - Slit width: 7 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 1 / Number real images: 7647 / Average electron dose: 40.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z
Z Y
Y X
X