+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Central part (C10) of bacteriophage SU10 capsid | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology | Protein of unknown function DUF5309 / Family of unknown function (DUF5309) / Major head protein Function and homology information Function and homology information | |||||||||
| Biological species |  Escherichia phage vB_EcoP_SU10 (virus) Escherichia phage vB_EcoP_SU10 (virus) | |||||||||
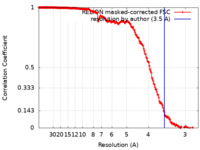
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.5 Å cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Siborova M / Fuzik T / Prochazkova M / Novacek J / Plevka P | |||||||||
| Funding support |  Czech Republic, 1 items Czech Republic, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Tail proteins of phage SU10 reorganize into the nozzle for genome delivery. Authors: Marta Šiborová / Tibor Füzik / Michaela Procházková / Jiří Nováček / Martin Benešík / Anders S Nilsson / Pavel Plevka /   Abstract: Escherichia coli phage SU10 belongs to the genus Kuravirus from the class Caudoviricetes of phages with short non-contractile tails. In contrast to other short-tailed phages, the tails of Kuraviruses ...Escherichia coli phage SU10 belongs to the genus Kuravirus from the class Caudoviricetes of phages with short non-contractile tails. In contrast to other short-tailed phages, the tails of Kuraviruses elongate upon cell attachment. Here we show that the virion of SU10 has a prolate head, containing genome and ejection proteins, and a tail, which is formed of portal, adaptor, nozzle, and tail needle proteins and decorated with long and short fibers. The binding of the long tail fibers to the receptors in the outer bacterial membrane induces the straightening of nozzle proteins and rotation of short tail fibers. After the re-arrangement, the nozzle proteins and short tail fibers alternate to form a nozzle that extends the tail by 28 nm. Subsequently, the tail needle detaches from the nozzle proteins and five types of ejection proteins are released from the SU10 head. The nozzle with the putative extension formed by the ejection proteins enables the delivery of the SU10 genome into the bacterial cytoplasm. It is likely that this mechanism of genome delivery, involving the formation of the tail nozzle, is employed by all Kuraviruses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14484.map.gz emd_14484.map.gz | 88.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14484-v30.xml emd-14484-v30.xml emd-14484.xml emd-14484.xml | 20.9 KB 20.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14484_fsc.xml emd_14484_fsc.xml | 18 KB | Display |  FSC data file FSC data file |
| Images |  emd_14484.png emd_14484.png | 268.3 KB | ||
| Others |  emd_14484_half_map_1.map.gz emd_14484_half_map_1.map.gz emd_14484_half_map_2.map.gz emd_14484_half_map_2.map.gz | 407 MB 407.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14484 http://ftp.pdbj.org/pub/emdb/structures/EMD-14484 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14484 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14484 | HTTPS FTP |
-Related structure data
| Related structure data |  7z45MC  7z44C  7z46C  7z47C  7z48C  7z49C  7z4aC  7z4bC  7z4fC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_14484.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14484.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.38 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_14484_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
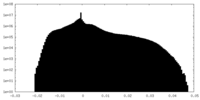
| Projections & Slices |
| ||||||||||||
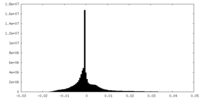
| Density Histograms |
-Half map: #1
| File | emd_14484_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
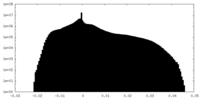
| Projections & Slices |
| ||||||||||||
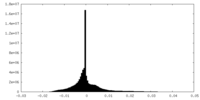
| Density Histograms |
- Sample components
Sample components
-Entire : Escherichia phage vB_EcoP_SU10
| Entire | Name:  Escherichia phage vB_EcoP_SU10 (virus) Escherichia phage vB_EcoP_SU10 (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Escherichia phage vB_EcoP_SU10
| Supramolecule | Name: Escherichia phage vB_EcoP_SU10 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 1519788 / Sci species name: Escherichia phage vB_EcoP_SU10 / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Molecular weight | Theoretical: 9.257 MDa |
-Macromolecule #1: Major head protein
| Macromolecule | Name: Major head protein / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Escherichia phage vB_EcoP_SU10 (virus) Escherichia phage vB_EcoP_SU10 (virus) |
| Molecular weight | Theoretical: 38.616457 KDa |
| Sequence | String: MANPTLFVSY DQNGKKLSFA NWISVLSPQD TPFVSMTGKE SINQTIFSWQ TDALASVDGN NAHVEGSRAE DGEMKPTVIK SNVTQILRK VVRVSDTANT TANYGRGREL MYQLEKKGKE IKRDLEKILL SGQARTDVLA DQYLTNSAAD PAVAGLNDTH A ARKTGAFQ ...String: MANPTLFVSY DQNGKKLSFA NWISVLSPQD TPFVSMTGKE SINQTIFSWQ TDALASVDGN NAHVEGSRAE DGEMKPTVIK SNVTQILRK VVRVSDTANT TANYGRGREL MYQLEKKGKE IKRDLEKILL SGQARTDVLA DQYLTNSAAD PAVAGLNDTH A ARKTGAFQ FLCAHGGLAG GVVDKTKNGP ADPDTGAVTV KVAQNASNPT TNIGFDEADI FDMTLQLYTA GSEADIIMIN PA HAKIFAG LQENTQGSRK RIFENTKQFI YEVNSITDPL GQSYKIIVNR WMPTDAVYFF RSADWTQMVL RAPKRTELAK DGS YEKWMI EMEVGLRHRN PYASGVLFTA AGKAAA |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: OTHER | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV | ||||||||||||
| Details | PFU 10^11 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.7 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 59000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.7 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Average exposure time: 1.0 sec. / Average electron dose: 49.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-7z45: |
 Movie
Movie Controller
Controller
















 Z
Z Y
Y X
X