[English] 日本語
 Yorodumi
Yorodumi- EMDB-10859: Cryo-EM structure of Tetrahymena thermophila mitochondrial ATP sy... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10859 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Tetrahymena thermophila mitochondrial ATP synthase - Fo-subcomplex | ||||||||||||
 Map data Map data | Local-resolution filtered full map of T. thermophila ATP synthase Fo-subcomplex | ||||||||||||
 Sample Sample |
| ||||||||||||
| Function / homology |  Function and homology information Function and homology informationmitochondrial proton-transporting ATP synthase complex, coupling factor F(o) / proton motive force-driven ATP synthesis / proton transmembrane transporter activity /  oxidoreductase activity / oxidoreductase activity /  hydrolase activity / hydrolase activity /  mitochondrion / mitochondrion /  membrane membraneSimilarity search - Function | ||||||||||||
| Biological species |   Tetrahymena thermophila (eukaryote) Tetrahymena thermophila (eukaryote) | ||||||||||||
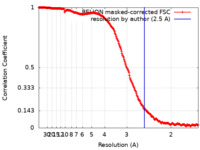
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.5 Å cryo EM / Resolution: 2.5 Å | ||||||||||||
 Authors Authors | Kock Flygaard R / Muhleip A / Amunts A | ||||||||||||
| Funding support |  Sweden, 3 items Sweden, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Type III ATP synthase is a symmetry-deviated dimer that induces membrane curvature through tetramerization. Authors: Rasmus Kock Flygaard / Alexander Mühleip / Victor Tobiasson / Alexey Amunts /  Abstract: Mitochondrial ATP synthases form functional homodimers to induce cristae curvature that is a universal property of mitochondria. To expand on the understanding of this fundamental phenomenon, we ...Mitochondrial ATP synthases form functional homodimers to induce cristae curvature that is a universal property of mitochondria. To expand on the understanding of this fundamental phenomenon, we characterized the unique type III mitochondrial ATP synthase in its dimeric and tetrameric form. The cryo-EM structure of a ciliate ATP synthase dimer reveals an unusual U-shaped assembly of 81 proteins, including a substoichiometrically bound ATPTT2, 40 lipids, and co-factors NAD and CoQ. A single copy of subunit ATPTT2 functions as a membrane anchor for the dimeric inhibitor IF. Type III specific linker proteins stably tie the ATP synthase monomers in parallel to each other. The intricate dimer architecture is scaffolded by an extended subunit-a that provides a template for both intra- and inter-dimer interactions. The latter results in the formation of tetramer assemblies, the membrane part of which we determined to 3.1 Å resolution. The structure of the type III ATP synthase tetramer and its associated lipids suggests that it is the intact unit propagating the membrane curvature. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10859.map.gz emd_10859.map.gz | 474.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10859-v30.xml emd-10859-v30.xml emd-10859.xml emd-10859.xml | 38 KB 38 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10859_fsc.xml emd_10859_fsc.xml | 21.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_10859.png emd_10859.png | 64.5 KB | ||
| Masks |  emd_10859_msk_1.map emd_10859_msk_1.map | 824 MB |  Mask map Mask map | |
| Others |  emd_10859_half_map_1.map.gz emd_10859_half_map_1.map.gz emd_10859_half_map_2.map.gz emd_10859_half_map_2.map.gz | 673.2 MB 670.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10859 http://ftp.pdbj.org/pub/emdb/structures/EMD-10859 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10859 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10859 | HTTPS FTP |
-Related structure data
| Related structure data |  6ynxMC  6ynvC  6ynwC  6ynyC  6ynzC  6yo0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10859.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10859.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local-resolution filtered full map of T. thermophila ATP synthase Fo-subcomplex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10859_msk_1.map emd_10859_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map 2
| File | emd_10859_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map 1
| File | emd_10859_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Mitochondrial ATP synthase, Fo-subcomplex
+Supramolecule #1: Mitochondrial ATP synthase, Fo-subcomplex
+Macromolecule #1: subunit a
+Macromolecule #2: subunit b
+Macromolecule #3: subunit d
+Macromolecule #4: subunit f
+Macromolecule #5: subunit i/j
+Macromolecule #6: subunit k
+Macromolecule #7: subunit 8
+Macromolecule #8: ATPTT3
+Macromolecule #9: ATPTT4
+Macromolecule #10: ATPTT5
+Macromolecule #11: ATPTT6
+Macromolecule #12: ATPTT7
+Macromolecule #13: ATPTT8
+Macromolecule #14: ATPTT9
+Macromolecule #15: ATPTT10
+Macromolecule #16: ATPTT11
+Macromolecule #17: ATPTT12
+Macromolecule #18: ATPTT13
+Macromolecule #19: ATPTT1
+Macromolecule #20: Inhibitor of F1 (IF1)
+Macromolecule #21: ATPTT2
+Macromolecule #22: CARDIOLIPIN
+Macromolecule #23: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
+Macromolecule #24: PHOSPHATE ION
+Macromolecule #25: Ubiquinone-8
+Macromolecule #26: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #27: MAGNESIUM ION
+Macromolecule #28: 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine
+Macromolecule #29: NICOTINAMIDE-ADENINE-DINUCLEOTIDE
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.75 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 165000 Bright-field microscopy / Nominal magnification: 165000 |
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average electron dose: 30.9 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























 Z
Z Y
Y X
X