[English] 日本語
 Yorodumi
Yorodumi- SASDBP9: Human Hsp90 co-chaperone Cdc37 protein (CD37) (Hsp90 co-chaperone... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: SASBDB / ID: SASDBP9 |
|---|---|
 Sample Sample | Human Hsp90 co-chaperone Cdc37 protein (CD37)
|
| Function / homology |  Function and homology information Function and homology informationregulation of type II interferon-mediated signaling pathway / HSP90-CDC37 chaperone complex / positive regulation of mitophagy in response to mitochondrial depolarization / protein kinase regulator activity / protein folding chaperone complex / post-transcriptional regulation of gene expression / Drug-mediated inhibition of ERBB2 signaling / Resistance of ERBB2 KD mutants to trastuzumab / Resistance of ERBB2 KD mutants to sapitinib / Resistance of ERBB2 KD mutants to tesevatinib ...regulation of type II interferon-mediated signaling pathway / HSP90-CDC37 chaperone complex / positive regulation of mitophagy in response to mitochondrial depolarization / protein kinase regulator activity / protein folding chaperone complex / post-transcriptional regulation of gene expression / Drug-mediated inhibition of ERBB2 signaling / Resistance of ERBB2 KD mutants to trastuzumab / Resistance of ERBB2 KD mutants to sapitinib / Resistance of ERBB2 KD mutants to tesevatinib / Resistance of ERBB2 KD mutants to neratinib / Resistance of ERBB2 KD mutants to osimertinib / Resistance of ERBB2 KD mutants to afatinib / Resistance of ERBB2 KD mutants to AEE788 / Resistance of ERBB2 KD mutants to lapatinib /  Drug resistance in ERBB2 TMD/JMD mutants / regulation of cyclin-dependent protein serine/threonine kinase activity / regulation of type I interferon-mediated signaling pathway / Drug resistance in ERBB2 TMD/JMD mutants / regulation of cyclin-dependent protein serine/threonine kinase activity / regulation of type I interferon-mediated signaling pathway /  protein targeting / RHOBTB2 GTPase cycle / Signaling by ERBB2 / protein targeting / RHOBTB2 GTPase cycle / Signaling by ERBB2 /  heat shock protein binding / Constitutive Signaling by Overexpressed ERBB2 / Signaling by ERBB2 TMD/JMD mutants / heat shock protein binding / Constitutive Signaling by Overexpressed ERBB2 / Signaling by ERBB2 TMD/JMD mutants /  Hsp90 protein binding / Constitutive Signaling by EGFRvIII / Signaling by ERBB2 ECD mutants / Signaling by ERBB2 KD Mutants / Regulation of necroptotic cell death / Downregulation of ERBB2 signaling / Hsp90 protein binding / Constitutive Signaling by EGFRvIII / Signaling by ERBB2 ECD mutants / Signaling by ERBB2 KD Mutants / Regulation of necroptotic cell death / Downregulation of ERBB2 signaling /  kinase binding / unfolded protein binding / kinase binding / unfolded protein binding /  protein folding / Constitutive Signaling by Ligand-Responsive EGFR Cancer Variants / protein-folding chaperone binding / protein folding / Constitutive Signaling by Ligand-Responsive EGFR Cancer Variants / protein-folding chaperone binding /  scaffold protein binding / protein stabilization / scaffold protein binding / protein stabilization /  protein kinase binding / extracellular exosome / protein kinase binding / extracellular exosome /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function |
| Biological species |   Homo sapiens (human) Homo sapiens (human) |
 Citation Citation |  Date: 2018 Mar Date: 2018 MarTitle: Disease Variants of FGFR3 Reveal Molecular Basis for the Recognition and Additional Roles for Cdc37 in Hsp90 Chaperone System Authors: Bunney T / Inglis A / Sanfelice D / Farrell B / Kerr C / Thompson G / Masson G / Thiyagarajan N / Svergun D / Williams R / Breeze A |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
-Data source
| SASBDB page |  SASDBP9 SASDBP9 |
|---|
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- External links
External links
| Related items in Molecule of the Month |
|---|
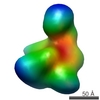
-Models
| Model #1123 |  Type: mix / Software: (ATSAS2.7.2) / Radius of dummy atoms: 1.90 A / Chi-square value: 1.88  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
|---|---|
| Model #1124 |  Type: dummy / Software: (ATSAS2.7.2) / Radius of dummy atoms: 3.00 A / Symmetry  : P1 / Chi-square value: 1.635 : P1 / Chi-square value: 1.635 Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
- Sample
Sample
 Sample Sample | Name: Human Hsp90 co-chaperone Cdc37 protein (CD37) / Specimen concentration: 1.10-4.20 |
|---|---|
| Buffer | Name: 25 mM Tris.Cl, 150 mM NaCl, 5% (v/v) glycerol, 1 mM TCEP pH: 8 |
| Entity #585 | Name: CDC37 / Type: protein / Description: Hsp90 co-chaperone Cdc37 / Formula weight: 44.426 / Num. of mol.: 1 / Source: Homo sapiens / References: UniProt: Q16543 / Type: protein / Description: Hsp90 co-chaperone Cdc37 / Formula weight: 44.426 / Num. of mol.: 1 / Source: Homo sapiens / References: UniProt: Q16543Sequence: MVDYSVWDHI EVSDDEDETH PNIDTASLFR WRHQARVERM EQFQKEKEEL DRGCRECKRK VAECQRKLKE LEVAEGGKAE LERLQAEAQQ LRKEERSWEQ KLEEMRKKEK SMPWNVDTLS KDGFSKSMVN TKPEKTEEDS EEVREQKHKT FVEKYEKQIK HFGMLRRWDD ...Sequence: MVDYSVWDHI EVSDDEDETH PNIDTASLFR WRHQARVERM EQFQKEKEEL DRGCRECKRK VAECQRKLKE LEVAEGGKAE LERLQAEAQQ LRKEERSWEQ KLEEMRKKEK SMPWNVDTLS KDGFSKSMVN TKPEKTEEDS EEVREQKHKT FVEKYEKQIK HFGMLRRWDD SQKYLSDNVH LVCEETANYL VIWCIDLEVE EKCALMEQVA HQTIVMQFIL ELAKSLKVDP RACFRQFFTK IKTADRQYME GFNDELEAFK ERVRGRAKLR IEKAMKEYEE EERKKRLGPG GLDPVEVYES LPEELQKCFD VKDVQMLQDA ISKMDPTDAK YHMQRCIDSG LWVPNSKASE AKEGEEAGPG DPLLEAVPKT GDEKDGSV |
-Experimental information
| Beam | Instrument name: PETRA III P12 / City: Hamburg / 国: Germany  / Type of source: X-ray synchrotron / Type of source: X-ray synchrotron Synchrotron / Wavelength: 0.124 Å / Dist. spec. to detc.: 3.1 mm Synchrotron / Wavelength: 0.124 Å / Dist. spec. to detc.: 3.1 mm | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: Pilatus 2M | ||||||||||||||||||||||||||||||||||||||||||
| Scan |
| ||||||||||||||||||||||||||||||||||||||||||
| Distance distribution function P(R) |
| ||||||||||||||||||||||||||||||||||||||||||
| Result |
|
 Movie
Movie Controller
Controller