+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8tj5 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
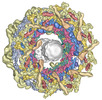
| Title | Inner spoke ring of the yeast NPC | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords |  TRANSPORT PROTEIN / TRANSPORT PROTEIN /  nuclear pore complex / nucleocytoplasmic transport / nuclear pore complex / nucleocytoplasmic transport /  nucleoporin / nucleoporin /  membrane protein / membrane protein /  translocase translocase | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to spindle checkpoint signaling / nuclear pore linkers / : / regulation of protein desumoylation / mRNA export from nucleus in response to heat stress / nuclear pore inner ring / protein localization to nuclear inner membrane / transcription-dependent tethering of RNA polymerase II gene DNA at nuclear periphery / nuclear pore central transport channel / telomere tethering at nuclear periphery ...response to spindle checkpoint signaling / nuclear pore linkers / : / regulation of protein desumoylation / mRNA export from nucleus in response to heat stress / nuclear pore inner ring / protein localization to nuclear inner membrane / transcription-dependent tethering of RNA polymerase II gene DNA at nuclear periphery / nuclear pore central transport channel / telomere tethering at nuclear periphery / regulation of nucleocytoplasmic transport / nuclear pore organization /  nuclear pore complex assembly / tRNA export from nucleus / post-transcriptional tethering of RNA polymerase II gene DNA at nuclear periphery / nuclear pore cytoplasmic filaments / nuclear pore nuclear basket / protein localization to kinetochore / structural constituent of nuclear pore / RNA export from nucleus / poly(A)+ mRNA export from nucleus / nucleocytoplasmic transport / nuclear pore complex assembly / tRNA export from nucleus / post-transcriptional tethering of RNA polymerase II gene DNA at nuclear periphery / nuclear pore cytoplasmic filaments / nuclear pore nuclear basket / protein localization to kinetochore / structural constituent of nuclear pore / RNA export from nucleus / poly(A)+ mRNA export from nucleus / nucleocytoplasmic transport /  nuclear localization sequence binding / regulation of mitotic nuclear division / NLS-bearing protein import into nucleus / nuclear localization sequence binding / regulation of mitotic nuclear division / NLS-bearing protein import into nucleus /  ribosomal small subunit export from nucleus / ribosomal small subunit export from nucleus /  ribosomal large subunit export from nucleus / mRNA transport / heterochromatin formation / ribosomal large subunit export from nucleus / mRNA transport / heterochromatin formation /  nuclear pore / nuclear periphery / nuclear pore / nuclear periphery /  chromosome segregation / promoter-specific chromatin binding / chromosome segregation / promoter-specific chromatin binding /  phospholipid binding / protein import into nucleus / phospholipid binding / protein import into nucleus /  protein transport / protein transport /  single-stranded DNA binding / single-stranded DNA binding /  nuclear envelope / nuclear envelope /  nuclear membrane / molecular adaptor activity / nuclear membrane / molecular adaptor activity /  cell cycle / cell cycle /  cell division / cell division /  chromatin binding / protein-containing complex binding / positive regulation of DNA-templated transcription / chromatin binding / protein-containing complex binding / positive regulation of DNA-templated transcription /  DNA binding / DNA binding /  RNA binding / identical protein binding / RNA binding / identical protein binding /  nucleus nucleusSimilarity search - Function | |||||||||||||||
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | |||||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 6.6 Å cryo EM / Resolution: 6.6 Å | |||||||||||||||
 Authors Authors | Akey, C.W. / Echeverria, I. / Ouch, C. / Fernandez-Martinez, J. / Rout, M.P. | |||||||||||||||
| Funding support |  United States, 4items United States, 4items
| |||||||||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: Implications of a multiscale structure of the yeast nuclear pore complex. Authors: Christopher W Akey / Ignacia Echeverria / Christna Ouch / Ilona Nudelman / Yi Shi / Junjie Wang / Brian T Chait / Andrej Sali / Javier Fernandez-Martinez / Michael P Rout /   Abstract: Nuclear pore complexes (NPCs) direct the nucleocytoplasmic transport of macromolecules. Here, we provide a composite multiscale structure of the yeast NPC, based on improved 3D density maps from ...Nuclear pore complexes (NPCs) direct the nucleocytoplasmic transport of macromolecules. Here, we provide a composite multiscale structure of the yeast NPC, based on improved 3D density maps from cryogenic electron microscopy and AlphaFold2 models. Key features of the inner and outer rings were integrated into a comprehensive model. We resolved flexible connectors that tie together the core scaffold, along with equatorial transmembrane complexes and a lumenal ring that anchor this channel within the pore membrane. The organization of the nuclear double outer ring reveals an architecture that may be shared with ancestral NPCs. Additional connections between the core scaffold and the central transporter suggest that under certain conditions, a degree of local organization is present at the periphery of the transport machinery. These connectors may couple conformational changes in the scaffold to the central transporter to modulate transport. Collectively, this analysis provides insights into assembly, transport, and NPC evolution. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8tj5.cif.gz 8tj5.cif.gz | 3.1 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8tj5.ent.gz pdb8tj5.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8tj5.json.gz 8tj5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tj/8tj5 https://data.pdbj.org/pub/pdb/validation_reports/tj/8tj5 ftp://data.pdbj.org/pub/pdb/validation_reports/tj/8tj5 ftp://data.pdbj.org/pub/pdb/validation_reports/tj/8tj5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  41300MC  8t9lC  8tieC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein/peptide , 1 types, 18 molecules 7384abkcmdefglhnji
| #1: Protein/peptide | Mass: 3166.895 Da / Num. of mol.: 18 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 |
|---|
-Protein , 10 types, 28 molecules Y0Z1ADGJBEHKCFILMONPQRSTUWVX
| #2: Protein | Mass: 169651.969 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / Strain: MATa ade2-1 ura3-1 his3-11 / References: UniProt: P38181 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / Strain: MATa ade2-1 ura3-1 his3-11 / References: UniProt: P38181#3: Protein | Mass: 156827.484 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / Strain: 15 trp1-1 leu2-3 / References: UniProt: P40064 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / Strain: 15 trp1-1 leu2-3 / References: UniProt: P40064#4: Protein | Mass: 86611.672 Da / Num. of mol.: 4 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / Strain: 112 can1-100 MLP1-PPX-ProteinA::HIS5 / References: UniProt: P14907 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / Strain: 112 can1-100 MLP1-PPX-ProteinA::HIS5 / References: UniProt: P14907#5: Protein | Mass: 57547.145 Da / Num. of mol.: 4 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: P48837 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: P48837#6: Protein | Mass: 49174.762 Da / Num. of mol.: 4 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: Q02199 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: Q02199#7: Protein | Mass: 191718.125 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: P47054 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: P47054#8: Protein | Mass: 188753.281 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: P52593 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: P52593#9: Protein | Mass: 96291.586 Da / Num. of mol.: 4 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: P34077 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: P34077#10: Protein | Mass: 52688.668 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: Q03790 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: Q03790#11: Protein |  / Nuclear pore protein NUP59 / Nuclear pore protein NUP59Mass: 58853.902 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: Q05166 Saccharomyces cerevisiae (brewer's yeast) / Cell line: W303 / References: UniProt: Q05166 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Nuclear Pore Complex Nuclear pore / Type: COMPLEX / Details: Protein A tagged Mlp1 pullout of NPC / Entity ID: all / Source: NATURAL Nuclear pore / Type: COMPLEX / Details: Protein A tagged Mlp1 pullout of NPC / Entity ID: all / Source: NATURAL |
|---|---|
| Molecular weight | Value: 18 MDa / Experimental value: YES |
| Source (natural) | Organism:   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast)Strain: MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 MLP1-PPX-ProteinA::HIS5 Cellular location: nuclear envelope / Organelle  : nucleus : nucleus |
| Buffer solution | pH: 7.5 Details: 20mM HEPES,50mM Potassium acetate,20mM NaCl,2mM MgCl2,1mM DTT |
| Specimen | Conc.: 0.1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES / Details: One step affinity purified : YES / Details: One step affinity purified |
Vitrification | Instrument: FEI VITROBOT MARK III / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 283 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS Details: Preliminary grid screening done manually with individual images of low magnification montages of candidate meshes. |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Calibrated magnification: 37651 X / Nominal defocus max: 3500 nm / Nominal defocus min: 1500 nm / Cs Bright-field microscopy / Calibrated magnification: 37651 X / Nominal defocus max: 3500 nm / Nominal defocus min: 1500 nm / Cs : 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE : 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 40 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 4015 / Details: 3218 images retained after triage |
| EM imaging optics | Energyfilter name : GIF Bioquantum / Energyfilter slit width: 20 eV : GIF Bioquantum / Energyfilter slit width: 20 eV |
| Image scans | Width: 3838 / Height: 3710 / Movie frames/image: 40 / Used frames/image: 2-40 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Details: CTF correction applied in RELION during the alignment and reconstruction Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 26049 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 6.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 208392 / Algorithm: FOURIER SPACE Details: Multibody extracted spoke image stack processed in CryoSPARC Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL Details: Rigid body docking of 3D map of Nucleoporins in the spoke complex with Chimera followed by flexible fitting and rebuilding in Coot. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Details: none / Source name: Other / Type: experimental model |
 Movie
Movie Controller
Controller











 PDBj
PDBj
