[English] 日本語
 Yorodumi
Yorodumi- EMDB-35329: Structure of mammalian spectrin-actin junctional complex of membr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of mammalian spectrin-actin junctional complex of membrane skeleton, Pointed-end segment, headpiece domain of dematin optimized | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of protein targeting to membrane / Gap junction degradation / Formation of annular gap junctions / Regulation of actin dynamics for phagocytic cup formation / EPHB-mediated forward signaling / VEGFA-VEGFR2 Pathway / Cell-extracellular matrix interactions / RHO GTPases Activate WASPs and WAVEs / MAP2K and MAPK activation /  Clathrin-mediated endocytosis ...negative regulation of protein targeting to membrane / Gap junction degradation / Formation of annular gap junctions / Regulation of actin dynamics for phagocytic cup formation / EPHB-mediated forward signaling / VEGFA-VEGFR2 Pathway / Cell-extracellular matrix interactions / RHO GTPases Activate WASPs and WAVEs / MAP2K and MAPK activation / Clathrin-mediated endocytosis ...negative regulation of protein targeting to membrane / Gap junction degradation / Formation of annular gap junctions / Regulation of actin dynamics for phagocytic cup formation / EPHB-mediated forward signaling / VEGFA-VEGFR2 Pathway / Cell-extracellular matrix interactions / RHO GTPases Activate WASPs and WAVEs / MAP2K and MAPK activation /  Clathrin-mediated endocytosis / spectrin-associated cytoskeleton / negative regulation of peptidyl-tyrosine phosphorylation / negative regulation of substrate adhesion-dependent cell spreading / cellular response to cytochalasin B / platelet dense tubular network membrane / regulation of transepithelial transport / structural constituent of postsynaptic actin cytoskeleton / morphogenesis of a polarized epithelium / negative regulation of focal adhesion assembly / postsynaptic actin cytoskeleton / protein localization to adherens junction / cell projection membrane / dense body / Tat protein binding / Clathrin-mediated endocytosis / spectrin-associated cytoskeleton / negative regulation of peptidyl-tyrosine phosphorylation / negative regulation of substrate adhesion-dependent cell spreading / cellular response to cytochalasin B / platelet dense tubular network membrane / regulation of transepithelial transport / structural constituent of postsynaptic actin cytoskeleton / morphogenesis of a polarized epithelium / negative regulation of focal adhesion assembly / postsynaptic actin cytoskeleton / protein localization to adherens junction / cell projection membrane / dense body / Tat protein binding /  regulation of filopodium assembly / apical protein localization / regulation of filopodium assembly / apical protein localization /  adherens junction assembly / positive regulation of fibroblast migration / adherens junction assembly / positive regulation of fibroblast migration /  regulation of lamellipodium assembly / regulation of lamellipodium assembly /  tight junction / RHO GTPases activate IQGAPs / RHO GTPases Activate Formins / regulation of norepinephrine uptake / tight junction / RHO GTPases activate IQGAPs / RHO GTPases Activate Formins / regulation of norepinephrine uptake /  lamellipodium assembly / regulation of synaptic vesicle endocytosis / lamellipodium assembly / regulation of synaptic vesicle endocytosis /  NuA4 histone acetyltransferase complex / apical junction complex / establishment or maintenance of cell polarity / NuA4 histone acetyltransferase complex / apical junction complex / establishment or maintenance of cell polarity /  spectrin binding / cortical cytoskeleton / negative regulation of peptidyl-threonine phosphorylation / spectrin binding / cortical cytoskeleton / negative regulation of peptidyl-threonine phosphorylation /  nitric-oxide synthase binding / erythrocyte development / nitric-oxide synthase binding / erythrocyte development /  kinesin binding / kinesin binding /  calyx of Held / calyx of Held /  brush border / actin filament bundle assembly / : / positive regulation of double-strand break repair via homologous recombination / positive regulation of blood coagulation / regulation of protein localization to plasma membrane / negative regulation of peptidyl-serine phosphorylation / cellular response to cAMP / cellular response to calcium ion / brush border / actin filament bundle assembly / : / positive regulation of double-strand break repair via homologous recombination / positive regulation of blood coagulation / regulation of protein localization to plasma membrane / negative regulation of peptidyl-serine phosphorylation / cellular response to cAMP / cellular response to calcium ion /  axonogenesis / axonogenesis /  cell motility / cell motility /  actin filament / regulation of actin cytoskeleton organization / actin filament / regulation of actin cytoskeleton organization /  adherens junction / adherens junction /  Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / Schaffer collateral - CA1 synapse / cytoplasmic ribonucleoprotein granule / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / Schaffer collateral - CA1 synapse / cytoplasmic ribonucleoprotein granule /  : / : /  nucleosome / nucleosome /  actin filament binding / actin filament binding /  actin cytoskeleton / actin cytoskeleton /  lamellipodium / regulation of cell shape / cytoplasmic vesicle / protein-containing complex assembly / lamellipodium / regulation of cell shape / cytoplasmic vesicle / protein-containing complex assembly /  postsynaptic density / postsynaptic density /  cytoskeleton / cytoskeleton /  hydrolase activity / hydrolase activity /  regulation of cell cycle / regulation of cell cycle /  ribonucleoprotein complex / ribonucleoprotein complex /  axon / axon /  signaling receptor binding / signaling receptor binding /  focal adhesion / glutamatergic synapse / focal adhesion / glutamatergic synapse /  synapse / synapse /  protein kinase binding / perinuclear region of cytoplasm / protein-containing complex / protein kinase binding / perinuclear region of cytoplasm / protein-containing complex /  ATP binding / ATP binding /  membrane / identical protein binding / membrane / identical protein binding /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Sus scrofa (pig) / Sus scrofa (pig) /   pig (pig) pig (pig) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.8 Å cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Li N / Chen S / Gao N | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2023 Journal: Cell / Year: 2023Title: Structural basis of membrane skeleton organization in red blood cells. Authors: Ningning Li / Siyi Chen / Kui Xu / Meng-Ting He / Meng-Qiu Dong / Qiangfeng Cliff Zhang / Ning Gao /  Abstract: The spectrin-based membrane skeleton is a ubiquitous membrane-associated two-dimensional cytoskeleton underneath the lipid membrane of metazoan cells. Mutations of skeleton proteins impair the ...The spectrin-based membrane skeleton is a ubiquitous membrane-associated two-dimensional cytoskeleton underneath the lipid membrane of metazoan cells. Mutations of skeleton proteins impair the mechanical strength and functions of the membrane, leading to several different types of human diseases. Here, we report the cryo-EM structures of the native spectrin-actin junctional complex (from porcine erythrocytes), which is a specialized short F-actin acting as the central organizational unit of the membrane skeleton. While an α-/β-adducin hetero-tetramer binds to the barbed end of F-actin as a flexible cap, tropomodulin and SH3BGRL2 together create an absolute cap at the pointed end. The junctional complex is strengthened by ring-like structures of dematin in the middle actin layers and by patterned periodic interactions with tropomyosin over its entire length. This work serves as a structural framework for understanding the assembly and dynamics of membrane skeleton and offers insights into mechanisms of various ubiquitous F-actin-binding factors in other F-actin systems. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35329.map.gz emd_35329.map.gz | 5.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35329-v30.xml emd-35329-v30.xml emd-35329.xml emd-35329.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35329.png emd_35329.png | 30.8 KB | ||
| Others |  emd_35329_half_map_1.map.gz emd_35329_half_map_1.map.gz emd_35329_half_map_2.map.gz emd_35329_half_map_2.map.gz | 40.7 MB 40.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35329 http://ftp.pdbj.org/pub/emdb/structures/EMD-35329 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35329 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35329 | HTTPS FTP |
-Related structure data
| Related structure data |  8ib2MC  8iahC  8iaiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35329.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35329.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.37 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_35329_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_35329_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Spectrin-actin junctional complex
| Entire | Name: Spectrin-actin junctional complex |
|---|---|
| Components |
|
-Supramolecule #1: Spectrin-actin junctional complex
| Supramolecule | Name: Spectrin-actin junctional complex / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Sus scrofa (pig) Sus scrofa (pig) |
-Macromolecule #1: Dematin actin binding protein
| Macromolecule | Name: Dematin actin binding protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   pig (pig) pig (pig) |
| Molecular weight | Theoretical: 45.569348 KDa |
| Sequence | String: MERLQKQPLT SPGSVSSSRG SSVPGSPSSI VAKMDNQVLG YKDLAAIPKD KAILDIERPD LMIYEPHFTY SLLEHVELPR SRERSLSPK STSPPPSPEV WAESRSPGTF PQASAPRTTG TPRTSLPHFH HPETTRPDSN IYKKPPIYKQ REPTGGSPQS K HLIEDLII ...String: MERLQKQPLT SPGSVSSSRG SSVPGSPSSI VAKMDNQVLG YKDLAAIPKD KAILDIERPD LMIYEPHFTY SLLEHVELPR SRERSLSPK STSPPPSPEV WAESRSPGTF PQASAPRTTG TPRTSLPHFH HPETTRPDSN IYKKPPIYKQ REPTGGSPQS K HLIEDLII ESSKFPAAQP PDPNQPAKIE TDYWPCPPSL AVVETEWRKR KASRRGAEEE EEEEDDDSGE EMKALRERQR EE LSKVTSN LGKMILKEEM EKSLPIRRKT RSLPDRTPFH TSLQAGTSKS SSLPAYGRTT LSRLQSTDFS PSGSETESPG LQN GEGQRG RMDRGTSLPC VLEQKIYPYE MLVVTNKGRT KLPPGVDRMR LERHLSAEDF SRVFSMSPEE FGKLALWKRN ELKK KASLF |
-Macromolecule #2: Actin, cytoplasmic 1
| Macromolecule | Name: Actin, cytoplasmic 1 / type: protein_or_peptide / ID: 2 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   pig (pig) pig (pig) |
| Molecular weight | Theoretical: 41.78266 KDa |
| Sequence | String: MDDDIAALVV DNGSGMCKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IEHGIVTNWD DMEKIWHHT FYNELRVAPE EHPVLLTEAP LNPKANREKM TQIMFETFNT PAMYVAIQAV LSLYASGRTT GIVMDSGDGV T HTVPIYEG ...String: MDDDIAALVV DNGSGMCKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IEHGIVTNWD DMEKIWHHT FYNELRVAPE EHPVLLTEAP LNPKANREKM TQIMFETFNT PAMYVAIQAV LSLYASGRTT GIVMDSGDGV T HTVPIYEG YALPHAILRL DLAGRDLTDY LMKILTERGY SFTTTAEREI VRDIKEKLCY VALDFEQEMA TAASSSSLEK SY ELPDGQV ITIGNERFRC PEALFQPSFL GMESCGIHET TFNSIMKCDV DIRKDLYANT VLSGGTTMYP GIADRMQKEI TAL APSTMK IKIIAPPERK YSVWIGGSIL ASLSTFQQMW ISKQEYDESG PSIVHRKCF |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 5 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 2.0 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 2.0 µm |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 34.4 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Initial angle assignment | Type: PROJECTION MATCHING |
|---|---|
| Final angle assignment | Type: PROJECTION MATCHING |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 60200 |
 Movie
Movie Controller
Controller









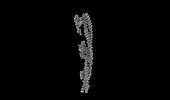



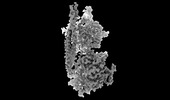


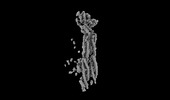


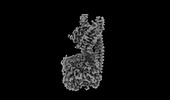



 Z
Z Y
Y X
X

















