+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
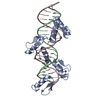
| Title | Cryo-EM structure of human cohesin-CTCF-DNA complex | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords |  Cohesin / NIPBL / Cohesin / NIPBL /  CTCF / CTCF /  DNA / chromosome folding / topologically associating domain / chromatin loops / DNA loop extrusion / sister chromatid cohesion / DNA / chromosome folding / topologically associating domain / chromatin loops / DNA loop extrusion / sister chromatid cohesion /  complex / complex /  ATPase / ATPase /  HEAT repeat protein / HEAT repeat protein /  DNA BINDING PROTEIN / DNA BINDING PROTEIN-DNA complex DNA BINDING PROTEIN / DNA BINDING PROTEIN-DNA complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationeye morphogenesis / external genitalia morphogenesis / gallbladder development / SMC loading complex / Scc2-Scc4 cohesin loading complex / ear morphogenesis / mitotic cohesin loading / response to DNA damage checkpoint signaling /  regulation of hair cycle / negative regulation of mitotic metaphase/anaphase transition ...eye morphogenesis / external genitalia morphogenesis / gallbladder development / SMC loading complex / Scc2-Scc4 cohesin loading complex / ear morphogenesis / mitotic cohesin loading / response to DNA damage checkpoint signaling / regulation of hair cycle / negative regulation of mitotic metaphase/anaphase transition ...eye morphogenesis / external genitalia morphogenesis / gallbladder development / SMC loading complex / Scc2-Scc4 cohesin loading complex / ear morphogenesis / mitotic cohesin loading / response to DNA damage checkpoint signaling /  regulation of hair cycle / negative regulation of mitotic metaphase/anaphase transition / chromatin insulator sequence binding / cohesin loader activity / positive regulation of sister chromatid cohesion / meiotic cohesin complex / Cohesin Loading onto Chromatin / maintenance of mitotic sister chromatid cohesion / regulation of hair cycle / negative regulation of mitotic metaphase/anaphase transition / chromatin insulator sequence binding / cohesin loader activity / positive regulation of sister chromatid cohesion / meiotic cohesin complex / Cohesin Loading onto Chromatin / maintenance of mitotic sister chromatid cohesion /  Establishment of Sister Chromatid Cohesion / forelimb morphogenesis / embryonic viscerocranium morphogenesis / establishment of meiotic sister chromatid cohesion / mitotic cohesin complex / Establishment of Sister Chromatid Cohesion / forelimb morphogenesis / embryonic viscerocranium morphogenesis / establishment of meiotic sister chromatid cohesion / mitotic cohesin complex /  cohesin complex / cohesin complex /  integrator complex / negative regulation of G2/M transition of mitotic cell cycle / uterus morphogenesis / regulation of centromeric sister chromatid cohesion / establishment of protein localization to chromatin / negative regulation of glial cell apoptotic process / regulation of developmental growth / integrator complex / negative regulation of G2/M transition of mitotic cell cycle / uterus morphogenesis / regulation of centromeric sister chromatid cohesion / establishment of protein localization to chromatin / negative regulation of glial cell apoptotic process / regulation of developmental growth /  genomic imprinting / embryonic digestive tract morphogenesis / replication-born double-strand break repair via sister chromatid exchange / cellular response to X-ray / lateral element / positive regulation of neuron migration / genomic imprinting / embryonic digestive tract morphogenesis / replication-born double-strand break repair via sister chromatid exchange / cellular response to X-ray / lateral element / positive regulation of neuron migration /  mediator complex binding / mediator complex binding /  chromo shadow domain binding / protein localization to chromosome, centromeric region / establishment of mitotic sister chromatid cohesion / chromatin looping / positive regulation of multicellular organism growth / metanephros development / positive regulation of ossification / digestive tract development / reciprocal meiotic recombination / embryonic forelimb morphogenesis / chromo shadow domain binding / protein localization to chromosome, centromeric region / establishment of mitotic sister chromatid cohesion / chromatin looping / positive regulation of multicellular organism growth / metanephros development / positive regulation of ossification / digestive tract development / reciprocal meiotic recombination / embryonic forelimb morphogenesis /  lncRNA binding / negative regulation of gene expression via chromosomal CpG island methylation / sister chromatid cohesion / face morphogenesis / negative regulation of interleukin-1 beta production / lncRNA binding / negative regulation of gene expression via chromosomal CpG island methylation / sister chromatid cohesion / face morphogenesis / negative regulation of interleukin-1 beta production /  microtubule motor activity / mitotic sister chromatid cohesion / stem cell population maintenance / dynein complex binding / microtubule motor activity / mitotic sister chromatid cohesion / stem cell population maintenance / dynein complex binding /  beta-tubulin binding / fat cell differentiation / mitotic spindle pole / outflow tract morphogenesis / beta-tubulin binding / fat cell differentiation / mitotic spindle pole / outflow tract morphogenesis /  regulation of DNA replication / mitotic sister chromatid segregation / somatic stem cell population maintenance / positive regulation of interleukin-10 production / regulation of DNA replication / mitotic sister chromatid segregation / somatic stem cell population maintenance / positive regulation of interleukin-10 production /  regulation of embryonic development / regulation of embryonic development /  chromosome, centromeric region / negative regulation of tumor necrosis factor production / chromosome, centromeric region / negative regulation of tumor necrosis factor production /  mitotic spindle assembly / developmental growth / localization / SUMOylation of DNA damage response and repair proteins / epigenetic regulation of gene expression / heart morphogenesis / condensed chromosome / protein localization to chromatin / Resolution of Sister Chromatid Cohesion / Meiotic synapsis / meiotic cell cycle / condensed nuclear chromosome / male germ cell nucleus / mitotic spindle assembly / developmental growth / localization / SUMOylation of DNA damage response and repair proteins / epigenetic regulation of gene expression / heart morphogenesis / condensed chromosome / protein localization to chromatin / Resolution of Sister Chromatid Cohesion / Meiotic synapsis / meiotic cell cycle / condensed nuclear chromosome / male germ cell nucleus /  chromosome segregation / chromosome segregation /  transcription coregulator binding / promoter-specific chromatin binding / sensory perception of sound / transcription coregulator binding / promoter-specific chromatin binding / sensory perception of sound /  brain development / response to radiation / brain development / response to radiation /  protein localization / protein localization /  kinetochore / kinetochore /  cognition / DNA-binding transcription repressor activity, RNA polymerase II-specific / cognition / DNA-binding transcription repressor activity, RNA polymerase II-specific /  nuclear matrix / nuclear matrix /  histone deacetylase binding / histone deacetylase binding /  spindle pole / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Separation of Sister Chromatids / transcription corepressor activity / double-strand break repair / spindle pole / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Separation of Sister Chromatids / transcription corepressor activity / double-strand break repair /  chromosome / mitotic cell cycle / midbody / chromosome / mitotic cell cycle / midbody /  double-stranded DNA binding double-stranded DNA bindingSimilarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 6.5 Å cryo EM / Resolution: 6.5 Å | ||||||||||||
 Authors Authors | Shi ZB / Bai XC | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2023 Journal: Mol Cell / Year: 2023Title: CTCF and R-loops are boundaries of cohesin-mediated DNA looping. Authors: Hongshan Zhang / Zhubing Shi / Edward J Banigan / Yoori Kim / Hongtao Yu / Xiao-Chen Bai / Ilya J Finkelstein /   Abstract: Cohesin and CCCTC-binding factor (CTCF) are key regulatory proteins of three-dimensional (3D) genome organization. Cohesin extrudes DNA loops that are anchored by CTCF in a polar orientation. Here, ...Cohesin and CCCTC-binding factor (CTCF) are key regulatory proteins of three-dimensional (3D) genome organization. Cohesin extrudes DNA loops that are anchored by CTCF in a polar orientation. Here, we present direct evidence that CTCF binding polarity controls cohesin-mediated DNA looping. Using single-molecule imaging, we demonstrate that a critical N-terminal motif of CTCF blocks cohesin translocation and DNA looping. The cryo-EM structure of the cohesin-CTCF complex reveals that this CTCF motif ahead of zinc fingers can only reach its binding site on the STAG1 cohesin subunit when the N terminus of CTCF faces cohesin. Remarkably, a C-terminally oriented CTCF accelerates DNA compaction by cohesin. DNA-bound Cas9 and Cas12a ribonucleoproteins are also polar cohesin barriers, indicating that stalling may be intrinsic to cohesin itself. Finally, we show that RNA-DNA hybrids (R-loops) block cohesin-mediated DNA compaction in vitro and are enriched with cohesin subunits in vivo, likely forming TAD boundaries. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32252.map.gz emd_32252.map.gz | 104.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32252-v30.xml emd-32252-v30.xml emd-32252.xml emd-32252.xml | 29.9 KB 29.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32252_fsc.xml emd_32252_fsc.xml | 12.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_32252.png emd_32252.png | 63.1 KB | ||
| Filedesc metadata |  emd-32252.cif.gz emd-32252.cif.gz | 11.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32252 http://ftp.pdbj.org/pub/emdb/structures/EMD-32252 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32252 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32252 | HTTPS FTP |
-Related structure data
| Related structure data |  7w1mMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32252.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32252.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.44 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
+Entire : Human cohesin-NIPBL-CTCF-DNA complex
+Supramolecule #1: Human cohesin-NIPBL-CTCF-DNA complex
+Supramolecule #2: Human cohesin-NIPBL-CTCF
+Supramolecule #3: DNA
+Macromolecule #1: Structural maintenance of chromosomes protein 1A
+Macromolecule #2: Structural maintenance of chromosomes protein 3
+Macromolecule #3: Double-strand-break repair protein rad21 homolog
+Macromolecule #4: Cohesin subunit SA-1
+Macromolecule #5: Nipped-B-like protein
+Macromolecule #8: Transcriptional repressor CTCF
+Macromolecule #6: DNA (118-MER)
+Macromolecule #7: DNA (118-MER)
+Macromolecule #9: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #10: BERYLLIUM TRIFLUORIDE ION
+Macromolecule #11: ZINC ION
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy Bright-field microscopy |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Average electron dose: 60.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||||||||
| Output model |  PDB-7w1m: |
 Movie
Movie Controller
Controller