[English] 日本語
 Yorodumi
Yorodumi- EMDB-27276: Saccharomyces cerevisiae Ufd1/Npl4/Cdc48 complex bound to two fol... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Saccharomyces cerevisiae Ufd1/Npl4/Cdc48 complex bound to two folded ubiquitin moieties and one unfolded ubiquitin in presence of SUMO-ubiquitin(K48polyUb)-mEOS and ATP, state 1 (uA) | |||||||||
 Map data Map data | composite map of the ubiquitin unfolded state 'uA' | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationSCF complex disassembly in response to cadmium stress / Ovarian tumor domain proteases / KEAP1-NFE2L2 pathway / endoplasmic reticulum membrane fusion / Cdc48p-Npl4p-Vms1p AAA ATPase complex / Doa10p ubiquitin ligase complex / ribophagy / DNA replication termination /  Neddylation / stress-induced homeostatically regulated protein degradation pathway ...SCF complex disassembly in response to cadmium stress / Ovarian tumor domain proteases / KEAP1-NFE2L2 pathway / endoplasmic reticulum membrane fusion / Cdc48p-Npl4p-Vms1p AAA ATPase complex / Doa10p ubiquitin ligase complex / ribophagy / DNA replication termination / Neddylation / stress-induced homeostatically regulated protein degradation pathway ...SCF complex disassembly in response to cadmium stress / Ovarian tumor domain proteases / KEAP1-NFE2L2 pathway / endoplasmic reticulum membrane fusion / Cdc48p-Npl4p-Vms1p AAA ATPase complex / Doa10p ubiquitin ligase complex / ribophagy / DNA replication termination /  Neddylation / stress-induced homeostatically regulated protein degradation pathway / positive regulation of mitochondrial fusion / sister chromatid biorientation / Neddylation / stress-induced homeostatically regulated protein degradation pathway / positive regulation of mitochondrial fusion / sister chromatid biorientation /  cytoplasm protein quality control by the ubiquitin-proteasome system / Hrd1p ubiquitin ligase ERAD-L complex / RQC complex / protein localization to vacuole / protein-containing complex disassembly / mitochondria-associated ubiquitin-dependent protein catabolic process / nuclear protein quality control by the ubiquitin-proteasome system / HSF1 activation / protein transport to vacuole involved in ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway / nonfunctional rRNA decay / protein phosphatase regulator activity / piecemeal microautophagy of the nucleus / mating projection tip / mitotic spindle disassembly / VCP-NPL4-UFD1 AAA ATPase complex / cytoplasm protein quality control by the ubiquitin-proteasome system / Hrd1p ubiquitin ligase ERAD-L complex / RQC complex / protein localization to vacuole / protein-containing complex disassembly / mitochondria-associated ubiquitin-dependent protein catabolic process / nuclear protein quality control by the ubiquitin-proteasome system / HSF1 activation / protein transport to vacuole involved in ubiquitin-dependent protein catabolic process via the multivesicular body sorting pathway / nonfunctional rRNA decay / protein phosphatase regulator activity / piecemeal microautophagy of the nucleus / mating projection tip / mitotic spindle disassembly / VCP-NPL4-UFD1 AAA ATPase complex /  replisome / replisome /  vesicle-fusing ATPase / vesicle-fusing ATPase /  : / ribosome-associated ubiquitin-dependent protein catabolic process / retrograde protein transport, ER to cytosol / nuclear outer membrane-endoplasmic reticulum membrane network / protein quality control for misfolded or incompletely synthesized proteins / autophagosome maturation / polyubiquitin modification-dependent protein binding / mRNA transport / rescue of stalled ribosome / : / ATP metabolic process / Neutrophil degranulation / : / ribosome-associated ubiquitin-dependent protein catabolic process / retrograde protein transport, ER to cytosol / nuclear outer membrane-endoplasmic reticulum membrane network / protein quality control for misfolded or incompletely synthesized proteins / autophagosome maturation / polyubiquitin modification-dependent protein binding / mRNA transport / rescue of stalled ribosome / : / ATP metabolic process / Neutrophil degranulation /  ubiquitin binding / ubiquitin binding /  macroautophagy / modification-dependent protein catabolic process / macroautophagy / modification-dependent protein catabolic process /  protein tag activity / positive regulation of protein localization to nucleus / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / protein tag activity / positive regulation of protein localization to nucleus / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process /  nuclear membrane / protein ubiquitination / nuclear membrane / protein ubiquitination /  ubiquitin protein ligase binding / endoplasmic reticulum membrane / perinuclear region of cytoplasm / ubiquitin protein ligase binding / endoplasmic reticulum membrane / perinuclear region of cytoplasm /  ATP hydrolysis activity / ATP hydrolysis activity /  mitochondrion / mitochondrion /  ATP binding / identical protein binding / ATP binding / identical protein binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | |||||||||
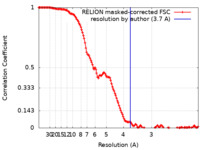
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.7 Å cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Lee HG / Lima CD | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: SUMO enhances unfolding of SUMO-polyubiquitin-modified substrates by the Ufd1/Npl4/Cdc48 complex. Authors: Hyein G Lee / Abigail A Lemmon / Christopher D Lima /  Abstract: The Ufd1/Npl4/Cdc48 complex is a universal protein segregase that plays key roles in eukaryotic cellular processes. Its functions orchestrating the clearance or removal of polyubiquitylated targets ...The Ufd1/Npl4/Cdc48 complex is a universal protein segregase that plays key roles in eukaryotic cellular processes. Its functions orchestrating the clearance or removal of polyubiquitylated targets are established; however, prior studies suggest that the complex also targets substrates modified by the ubiquitin-like protein SUMO. Here, we show that interactions between Ufd1 and SUMO enhance unfolding of substrates modified by SUMO-polyubiquitin hybrid chains by the budding yeast Ufd1/Npl4/Cdc48 complex compared to substrates modified by polyubiquitin chains, a difference that is accentuated when the complex has a choice between these substrates. Incubating Ufd1/Npl4/Cdc48 with a substrate modified by a SUMO-polyubiquitin hybrid chain produced a series of single-particle cryo-EM structures that reveal features of interactions between Ufd1/Npl4/Cdc48 and ubiquitin prior to and during unfolding of ubiquitin. These results are consistent with cellular functions for SUMO and ubiquitin modifications and support a physical model wherein Ufd1/Npl4/Cdc48, SUMO, and ubiquitin conjugation pathways converge to promote clearance of proteins modified with SUMO and polyubiquitin. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27276.map.gz emd_27276.map.gz | 32.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27276-v30.xml emd-27276-v30.xml emd-27276.xml emd-27276.xml | 54.1 KB 54.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27276_fsc.xml emd_27276_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_27276.png emd_27276.png | 88.6 KB | ||
| Others |  emd_27276_additional_1.map.gz emd_27276_additional_1.map.gz emd_27276_additional_10.map.gz emd_27276_additional_10.map.gz emd_27276_additional_11.map.gz emd_27276_additional_11.map.gz emd_27276_additional_12.map.gz emd_27276_additional_12.map.gz emd_27276_additional_13.map.gz emd_27276_additional_13.map.gz emd_27276_additional_2.map.gz emd_27276_additional_2.map.gz emd_27276_additional_3.map.gz emd_27276_additional_3.map.gz emd_27276_additional_4.map.gz emd_27276_additional_4.map.gz emd_27276_additional_5.map.gz emd_27276_additional_5.map.gz emd_27276_additional_6.map.gz emd_27276_additional_6.map.gz emd_27276_additional_7.map.gz emd_27276_additional_7.map.gz emd_27276_additional_8.map.gz emd_27276_additional_8.map.gz emd_27276_additional_9.map.gz emd_27276_additional_9.map.gz emd_27276_half_map_1.map.gz emd_27276_half_map_1.map.gz emd_27276_half_map_2.map.gz emd_27276_half_map_2.map.gz | 17.5 MB 13.4 MB 171.5 MB 9.7 MB 171.1 MB 171.3 MB 171.3 MB 171.1 MB 171 MB 171.5 MB 171 MB 12.7 MB 10.2 MB 171.5 MB 171.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27276 http://ftp.pdbj.org/pub/emdb/structures/EMD-27276 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27276 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27276 | HTTPS FTP |
-Related structure data
| Related structure data |  8dauMC  8darC  8dasC  8datC  8davC  8dawC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27276.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27276.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | composite map of the ubiquitin unfolded state 'uA' | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.064 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
+Additional map: overall refinement post-processed map of the ubiquitin unfolded...
+Additional map: post-processed focused refinement map of the D1/D2 domains...
+Additional map: focused refinement half map 1 of Cdc48 of...
+Additional map: post-processed focused refinement map of Npl4 and polyubiquitin...
+Additional map: focused refinement half map 1 of Npl4 and...
+Additional map: focused refinement half map 2 of the D1/D2...
+Additional map: focused refinement half map 1 of the D1/D2...
+Additional map: focused refinement half map 1 of the central...
+Additional map: focused refinement half map 2 of Npl4/polyubiquitin density...
+Additional map: focused refinement half map 2 of Cdc48 of...
+Additional map: focused refinement half map 2 of the central...
+Additional map: post-processed focused refinement map of Cdc48 of the...
+Additional map: post-processed focused refinement map of the central Ufd1/Npl4/polyubiquitin...
+Half map: overall refinement half map 1
+Half map: overall refinement half map 2 of the ubiquitin unfolded state 'uA'
- Sample components
Sample components
+Entire : Saccharomyces cerevisiae Ufd1/Npl4/Cdc48 complex bound to two Ubi...
+Supramolecule #1: Saccharomyces cerevisiae Ufd1/Npl4/Cdc48 complex bound to two Ubi...
+Supramolecule #2: Saccharomyces cerevisiae Ufd1/Npl4/Cdc48 complex bound to two ubi...
+Supramolecule #3: Cdc48 hexamer
+Supramolecule #4: Two folded ubiqutin moieties and one unfolded ubiquitin bound to ...
+Supramolecule #5: D1/D2 ATPase domains of the Cdc48 hexamer
+Supramolecule #6: Two folded ubiqutin moieties bound to Npl4
+Macromolecule #1: Cell division control protein 48
+Macromolecule #2: Nuclear protein localization protein 4
+Macromolecule #3: Ubiquitin fusion degradation protein 1
+Macromolecule #4: Ubiquitin
+Macromolecule #5: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #6: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #7: ZINC ION
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 20 mM HEPES pH 8.0, 150 mM NaCl, 0.1 mM TCEP, 1 mM MgCl2, 5 mM ATP. Added 0.05% CHAPSO before vitrification. |
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV / Details: 8 s wait, 4 s blot before plunging. |
| Details | Ufd1/Npl4/Cdc48 was pre-incubated with SUMO-ubiquitin(K48polyUb)-mEOS and ATP |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.2 µm Bright-field microscopy / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.2 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 70.577 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z
Z Y
Y X
X